Public versus Private Drug Insurance and Outcomes of Patients Requiring Biologic Therapies for Inflammatory Bowel Disease
- PMID: 28239601
- PMCID: PMC5292380
- DOI: 10.1155/2017/7365937
Public versus Private Drug Insurance and Outcomes of Patients Requiring Biologic Therapies for Inflammatory Bowel Disease
Abstract
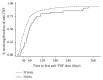
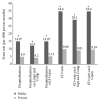
Background. Antitumor necrosis factor (anti-TNF) therapy is a highly effective but costly treatment for inflammatory bowel disease (IBD). Methods. We conducted a retrospective cohort study of IBD patients who were prescribed anti-TNF therapy (2007-2014) in Ontario. We assessed if the insurance type was a predictor of timely access to anti-TNF therapy and nonroutine health utilization (emergency department visits and hospitalizations). Results. There were 268 patients with IBD who were prescribed anti-TNF therapy. Public drug coverage was associated with longer median wait times to first dose than private one (56 versus 35 days, P = 0.002). After adjusting for confounders, publicly insured patients were less likely to receive timely access to anti-TNF therapy compared with those privately insured (adjusted hazard ratio, 0.66; 95% CI: 0.45-0.95). After adjustment for demographic and clinical characteristics, publicly funded subjects were more than 2-fold more likely to require hospitalization (incidence rate ratio [IRR], 2.30; 95% CI: 1.19-4.43) and ED visits (IRR 2.42; 95% CI: 1.44-4.08) related to IBD. Conclusions. IBD patients in Ontario with public drug coverage experienced greater delays in access to anti-TNF therapy than privately insured patients and have a higher rate of hospitalizations and ED visits related to IBD.
Conflict of interest statement
K. Croitoru received educational grants from Janssen, Abbvie, and Takeda and has served on advisory boards for Abbvie and Takeda. A. H. Steinhart received research grants from Abbvie, Amgen, Pfizer, and Millennium and speaking honoraria from Abbvie, Janssen, Takeda, and Shire; he has served on advisory boards for Abbvie, Actavis, Janssen, Takeda, Pharmascience, and Shire. M. S. Silverberg received research support and consulting fees from Janssen, Abbvie, Takeda, and Prometheus.
Figures
Similar articles
-
The effects of race and socioeconomic status on immunomodulator and anti-tumor necrosis factor use among ambulatory patients with inflammatory bowel disease in the United States.Am J Gastroenterol. 2013 Dec;108(12):1824-30. doi: 10.1038/ajg.2013.192. Am J Gastroenterol. 2013. PMID: 24300857
-
Comparative risk of incident venous thromboembolism in patients with inflammatory bowel disease initiating tumour necrosis factor-α inhibitors or nonbiologic agents: a cohort study.CMAJ. 2017 Nov 27;189(47):E1438-E1447. doi: 10.1503/cmaj.161485. CMAJ. 2017. PMID: 29180383 Free PMC article.
-
The Effect of Initiation of Anti-TNF Therapy on the Subsequent Direct Health Care Costs of Inflammatory Bowel Disease.Inflamm Bowel Dis. 2019 Sep 18;25(10):1718-1728. doi: 10.1093/ibd/izz063. Inflamm Bowel Dis. 2019. PMID: 31211836
-
Preoperative use of anti-TNF therapy and postoperative complications in inflammatory bowel diseases: a meta-analysis.J Crohns Colitis. 2013 Dec;7(11):853-67. doi: 10.1016/j.crohns.2013.01.014. Epub 2013 Mar 20. J Crohns Colitis. 2013. PMID: 23523418 Review.
-
Balancing the risks and benefits of biologic therapy in inflammatory bowel diseases.Expert Opin Drug Saf. 2015;14(12):1915-34. doi: 10.1517/14740338.2015.1108961. Epub 2015 Nov 11. Expert Opin Drug Saf. 2015. PMID: 26559664 Review.
Cited by
-
Accelerating Earlier Access to Anti-TNF-α Agents with Biosimilar Medicines in the Management of Inflammatory Bowel Disease.J Clin Med. 2025 Feb 26;14(5):1561. doi: 10.3390/jcm14051561. J Clin Med. 2025. PMID: 40095484 Free PMC article. Review.
-
Diagnostic Delay of Inflammatory Bowel Disease Is Significantly Higher in Public versus Private Health Care System in Mexican Patients.Inflamm Intest Dis. 2021 Dec 6;7(2):72-80. doi: 10.1159/000520522. eCollection 2022 Jul. Inflamm Intest Dis. 2021. PMID: 35979192 Free PMC article.
-
Insurance Denial of Biologic Therapy is Associated With Reduced Remission Rates in Inflammatory Bowel Disease Patients.Gastro Hep Adv. 2025 Feb 27;4(6):100647. doi: 10.1016/j.gastha.2025.100647. eCollection 2025. Gastro Hep Adv. 2025. PMID: 40692821 Free PMC article.
-
Patterns and predictors of long-term retention of inflammatory bowel or rheumatoid disease patients on innovator infliximab: an analysis of a Canadian prescriptions claims database.Patient Prefer Adherence. 2018 Sep 18;12:1805-1814. doi: 10.2147/PPA.S171363. eCollection 2018. Patient Prefer Adherence. 2018. PMID: 30271124 Free PMC article.
-
Delays Related to Prior Authorization in Inflammatory Bowel Disease.Pediatrics. 2022 Mar 1;149(3):e2021052501. doi: 10.1542/peds.2021-052501. Pediatrics. 2022. PMID: 35190811 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

