Engraftment of Human Primary Acute Myeloid Leukemia Defined by Integrated Genetic Profiling in NOD/SCID/IL2rγnull Mice for Preclinical Ceramide-Based Therapeutic Evaluation
- PMID: 28239612
- PMCID: PMC5321614
- DOI: 10.4172/2329-6917.1000146
Engraftment of Human Primary Acute Myeloid Leukemia Defined by Integrated Genetic Profiling in NOD/SCID/IL2rγnull Mice for Preclinical Ceramide-Based Therapeutic Evaluation
Abstract
Acute Myeloid Leukemia (AML) is a highly heterogeneous and poor prognosis disease with few available therapeutic options. Novel advances are urgently needed, however effective models to test experimental therapeutics have been lacking. Recently, NOD/SCID/IL2rγnull (NSG) mice were shown to engraft primary human AML in a manner that recapitulated the natural disease and its progression. Additionally, integrated genomic profiling was used to refine risk stratification of AML. In this study, we demonstrated the engraftment of molecularly defined primary AML in NSG mice. We showed that AML that express DNMT3A mutations, which predict for adverse outcome, engrafted with exceptional efficacy. Lastly, we demonstrated that human AML-engrafted NSG mice can be effectively used to study novel ceramide-based therapeutics. Ceramide is a bioactive sphingolipid that has been implicated as an inducer of apoptosis. Elevation in cancer cell ceramide levels either via exogenous delivery or by provoking intracellular ceramide generation is the goal of ceramide-based therapeutics. In this study, we used the human AML-engrafted NSG mouse model to evaluate nanoliposomal short-chain C6-ceramide and a nanoliposomal formulation of the ceramide-inducer tamoxifen. Altogether, the NSG model is likely to prove invaluable in the study of novel agents, sushc as ceramide-based therapeutics, with the ability to define therapeutic activity against specific molecularly defined and risk stratified AML.
Keywords: Acute myeloid leukemia; Ceramide; DNMT3A; Integrated genetic profiling; NSG mouse; Nanoliposome; Tamoxifen.
Figures
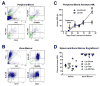
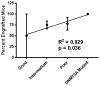
References
-
- Baldus CD, Mrózek K, Marcucci G, Bloomfield CD. Clinical outcome of de novo acute myeloid leukaemia patients with normal cytogenetics is affected by molecular genetic alterations: a concise review. Br J Haematol. 2007;137:387–400. - PubMed
-
- Shih AH, Abdel-Wahab O, Patel JP, Levine RL. The role of mutations in epigenetic regulators in myeloid malignancies. Nat Rev Cancer. 2012;12:599–612. - PubMed
-
- Agliano A, Martin-Padura I, Mancuso P, Marighetti P, Rabascio C, et al. Human acute leukemia cells injected in NOD/LtSz-scid/IL-2Rgamma null mice generate a faster and more efficient disease compared to other NOD/scid-related strains. Int J Cancer. 2008;123:2222–2227. - PubMed
-
- Ishikawa F, Yoshida S, Saito Y, Hijikata A, Kitamura H, et al. Chemotherapy-resistant human AML stem cells home to and engraft within the bone-marrow endosteal region. Nat Biotechnol. 2007;25:1315–1321. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
