Alzheimer's disease: The influence of age on clinical heterogeneity through the human brain connectome
- PMID: 28239637
- PMCID: PMC5318292
- DOI: 10.1016/j.dadm.2016.12.007
Alzheimer's disease: The influence of age on clinical heterogeneity through the human brain connectome
Abstract
Introduction: One major factor that influences the heterogeneity of Alzheimer's disease (AD) is age: younger AD patients more frequently exhibit atypical forms of AD. We propose that this age-related heterogeneity can be understood better by considering age-related differences in atrophy in the context of large-scale brain networks subserving cognitive functions that contribute to memory.
Methods: We examined data from 75 patients with mild AD dementia from Alzheimer's Disease Neuroimaging Initiative. These individuals were chosen because they have cerebrospinal fluid amyloid and p-tau levels in the range suggesting the presence of AD neuropathology, and because they were either younger than age 65 years early-onset AD (EOAD) or age 80 years or older late-onset AD (LOAD).
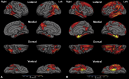
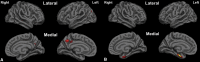
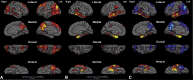
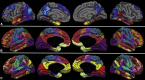
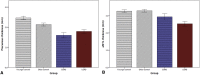
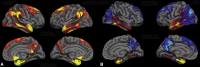
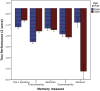
Results: In the EOAD group, the most prominent atrophy was present in the posterior cingulate cortex, whereas in the LOAD group, atrophy was most prominent in the medial temporal lobe. Structural covariance analysis showed that the magnitude of atrophy in these epicenters is strongly correlated with a distributed atrophy pattern similar to distinct intrinsic connectivity networks in the healthy brain. An examination of memory performance in EOAD dementia versus LOAD dementia demonstrated relatively more prominent impairment in encoding in the EOAD group than in the LOAD group, with similar performance in memory storage in LOAD and EOAD but greater impairment in semantic memory in LOAD than in EOAD.
Discussion: The observations provide novel insights about age as a major factor contributing to the heterogeneity in the topography of AD-related cortical atrophy.
Keywords: Age; Alzheimer's disease; Cortical thickness; MRI; Memory.
Figures
Similar articles
-
Early-onset and late-onset Alzheimer's disease are associated with distinct patterns of memory impairment.Cortex. 2016 Jan;74:217-32. doi: 10.1016/j.cortex.2015.10.014. Epub 2015 Nov 17. Cortex. 2016. PMID: 26694580
-
Difference in imaging biomarkers of neurodegeneration between early and late-onset amnestic Alzheimer's disease.Neurobiol Aging. 2017 Jun;54:22-30. doi: 10.1016/j.neurobiolaging.2017.02.010. Epub 2017 Feb 21. Neurobiol Aging. 2017. PMID: 28314160
-
The APOE4 effect: structural brain differences in Alzheimer's disease according to the age at symptom onset.Eur J Neurol. 2023 Mar;30(3):597-605. doi: 10.1111/ene.15657. Epub 2022 Dec 20. Eur J Neurol. 2023. PMID: 36463489 Free PMC article.
-
Early-onset Alzheimer's disease: nonamnestic subtypes and type 2 AD.Arch Med Res. 2012 Nov;43(8):677-85. doi: 10.1016/j.arcmed.2012.11.009. Epub 2012 Nov 21. Arch Med Res. 2012. PMID: 23178565 Free PMC article. Review.
-
Disease tracking markers for Alzheimer's disease at the prodromal (MCI) stage.J Alzheimers Dis. 2011;26 Suppl 3:159-99. doi: 10.3233/JAD-2011-0043. J Alzheimers Dis. 2011. PMID: 21971460 Review.
Cited by
-
Posterior cortical atrophy phenotypic heterogeneity revealed by decoding 18F-FDG-PET.Brain Commun. 2021 Aug 19;3(4):fcab182. doi: 10.1093/braincomms/fcab182. eCollection 2021. Brain Commun. 2021. PMID: 34805993 Free PMC article.
-
Medial Temporal Atrophy in Posterior Cortical Atrophy and Its Relationship to the Cingulate Island Sign.J Alzheimers Dis. 2022;86(1):491-498. doi: 10.3233/JAD-215263. J Alzheimers Dis. 2022. PMID: 35068459 Free PMC article.
-
Differential Involvement of the Locus Coeruleus in Early- and Late-Onset Alzheimer's Disease: A Potential Mechanism of Clinical Differences?J Geriatr Psychiatry Neurol. 2022 Sep;35(5):733-739. doi: 10.1177/08919887211044755. Epub 2021 Sep 9. J Geriatr Psychiatry Neurol. 2022. PMID: 34496652 Free PMC article.
-
The role of age on tau PET uptake and gray matter atrophy in atypical Alzheimer's disease.Alzheimers Dement. 2019 May;15(5):675-685. doi: 10.1016/j.jalz.2018.12.016. Epub 2019 Mar 8. Alzheimers Dement. 2019. PMID: 30853465 Free PMC article.
-
M1 muscarinic acetylcholine receptor dysfunction in moderate Alzheimer's disease pathology.Brain Commun. 2020;2(2):fcaa058. doi: 10.1093/braincomms/fcaa058. Epub 2020 May 12. Brain Commun. 2020. PMID: 32766549 Free PMC article.
References
-
- McKhann G., Drachman D., Folstein M., Katzman R., Price D., Stadlan E.M. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. - PubMed
-
- Becker J.T., Huff F.J., Nebes R.D., Holland A., Boller F. Neuropsychological function in Alzheimer's disease. Pattern of impairment and rates of progression. Arch Neurol. 1988;45:263–268. - PubMed
-
- Martin A., Brouwers P., Lalonde F., Cox C., Teleska P., Fedio P. Towards a behavioral typology of Alzheimer's patients. J Clin Exp Neuropsychol. 1986;8:594–610. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
