Mammary adipocytes stimulate breast cancer invasion through metabolic remodeling of tumor cells
- PMID: 28239646
- PMCID: PMC5313068
- DOI: 10.1172/jci.insight.87489
Mammary adipocytes stimulate breast cancer invasion through metabolic remodeling of tumor cells
Abstract
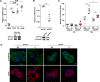
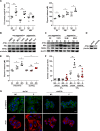
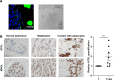
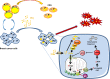
In breast cancer, a key feature of peritumoral adipocytes is their loss of lipid content observed both in vitro and in human tumors. The free fatty acids (FFAs), released by adipocytes after lipolysis induced by tumor secretions, are transferred and stored in tumor cells as triglycerides in lipid droplets. In tumor cell lines, we demonstrate that FFAs can be released over time from lipid droplets through an adipose triglyceride lipase-dependent (ATGL-dependent) lipolytic pathway. In vivo, ATGL is expressed in human tumors where its expression correlates with tumor aggressiveness and is upregulated by contact with adipocytes. The released FFAs are then used for fatty acid β-oxidation (FAO), an active process in cancer but not normal breast epithelial cells, and regulated by coculture with adipocytes. However, in cocultivated cells, FAO is uncoupled from ATP production, leading to AMPK/acetyl-CoA carboxylase activation, a circle that maintains this state of metabolic remodeling. The increased invasive capacities of tumor cells induced by coculture are completely abrogated by inhibition of the coupled ATGL-dependent lipolysis/FAO pathways. These results show a complex metabolic symbiosis between tumor-surrounding adipocytes and cancer cells that stimulate their invasiveness, highlighting ATGL as a potential therapeutic target to impede breast cancer progression.
Conflict of interest statement
Conflict of interest: The authors have declared that no conflict of interest exists.
Figures
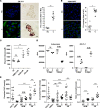
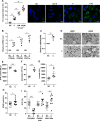
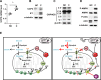
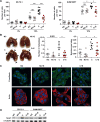
References
-
- Jose C, Bellance N, Rossignol R. Choosing between glycolysis and oxidative phosphorylation: a tumor’s dilemma? Biochim Biophys Acta. 2011;1807(6):552–561. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

