Loss of immune homeostasis dictates SHIV rebound after stem-cell transplantation
- PMID: 28239658
- PMCID: PMC5322807
- DOI: 10.1172/jci.insight.91230
Loss of immune homeostasis dictates SHIV rebound after stem-cell transplantation
Abstract
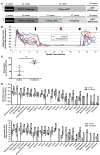
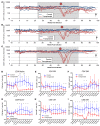
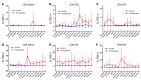
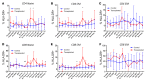
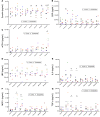
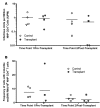
The conditioning regimen used as part of the Berlin patient's hematopoietic cell transplant likely contributed to his eradication of HIV infection. We studied the impact of conditioning in simian-human immunodeficiency virus-infected (SHIV-infected) macaques suppressed by combination antiretroviral therapy (cART). The conditioning regimen resulted in a dramatic, but incomplete depletion of CD4+ and CD8+ T cells and CD20+ B cells, increased T cell activation and exhaustion, and a significant loss of SHIV-specific Abs. The disrupted T cell homeostasis and markers of microbial translocation positively correlated with an increased viral rebound after cART interruption. Quantitative viral outgrowth and Tat/rev-induced limiting dilution assays showed that the size of the latent SHIV reservoir did not correlate with viral rebound. These findings identify perturbations of the immune system as a mechanism for the failure of autologous transplantation to eradicate HIV. Thus, transplantation strategies may be improved by incorporating immune modulators to prevent disrupted homeostasis, and gene therapy to protect transplanted cells.
Conflict of interest statement
Conflict of interest: The authors have declared that no conflict of interest exists.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

