α2-Adrenergic blockade rescues hypoglossal motor defense against obstructive sleep apnea
- PMID: 28239660
- PMCID: PMC5313063
- DOI: 10.1172/jci.insight.91456
α2-Adrenergic blockade rescues hypoglossal motor defense against obstructive sleep apnea
Abstract
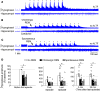
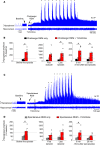
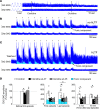
Decreased noradrenergic excitation of hypoglossal motoneurons during sleep causing hypotonia of pharyngeal dilator muscles is a major contributor to the pathogenesis of obstructive sleep apnea (OSA), a widespread disease for which treatment options are limited. Previous OSA drug candidates targeting various excitatory/inhibitory receptors on hypoglossal motoneurons have proved unviable in reactivating these neurons, particularly during rapid-eye-movement (REM) sleep. To identify a viable drug target, we show that the repurposed α2-adrenergic antagonist yohimbine potently reversed the depressant effect of REM sleep on baseline hypoglossal motoneuron activity (a first-line motor defense against OSA) in rats. Remarkably, yohimbine also restored the obstructive apnea-induced long-term facilitation of hypoglossal motoneuron activity (hLTF), a much-neglected form of noradrenergic-dependent neuroplasticity that could provide a second-line motor defense against OSA but was also depressed during REM sleep. Corroborating immunohistologic, optogenetic, and pharmacologic evidence confirmed that yohimbine's beneficial effects on baseline hypoglossal motoneuron activity and hLTF were mediated mainly through activation of pontine A7 and A5 noradrenergic neurons. Our results suggest a 2-tier (impaired first- and second-line motor defense) mechanism of noradrenergic-dependent pathogenesis of OSA and a promising pharmacotherapy for rescuing both these intrinsic defenses against OSA through disinhibition of A7 and A5 neurons by α2-adrenergic blockade.
Conflict of interest statement
Conflict of interest: The authors have declared that no conflict of interest exists.
Figures



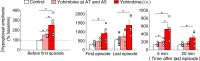
Similar articles
-
Activity of pontine A7 noradrenergic neurons is suppressed during REM sleep.J Appl Physiol (1985). 2022 Jul 1;133(1):130-143. doi: 10.1152/japplphysiol.00771.2021. Epub 2022 May 26. J Appl Physiol (1985). 2022. PMID: 35616303 Free PMC article.
-
Computational model of brain-stem circuit for state-dependent control of hypoglossal motoneurons.J Neurophysiol. 2018 Jul 1;120(1):296-305. doi: 10.1152/jn.00728.2017. Epub 2018 Apr 4. J Neurophysiol. 2018. PMID: 29617218 Free PMC article.
-
Muscarinic Inhibition of Hypoglossal Motoneurons: Possible Implications for Upper Airway Muscle Hypotonia during REM Sleep.J Neurosci. 2019 Oct 2;39(40):7910-7919. doi: 10.1523/JNEUROSCI.0461-19.2019. Epub 2019 Aug 16. J Neurosci. 2019. PMID: 31420456 Free PMC article.
-
Noradrenergic modulation of hypoglossal motoneuron excitability: developmental and putative state-dependent mechanisms.Arch Ital Biol. 2011 Dec 1;149(4):426-53. doi: 10.4449/aib.v149i4.1271. Arch Ital Biol. 2011. PMID: 22205594 Review.
-
Revisiting Antagonist Effects in Hypoglossal Nucleus: Brainstem Circuit for the State-Dependent Control of Hypoglossal Motoneurons: A Hypothesis.Front Neurol. 2015 Dec 1;6:254. doi: 10.3389/fneur.2015.00254. eCollection 2015. Front Neurol. 2015. PMID: 26648908 Free PMC article. Review.
Cited by
-
Pharmacotherapy of Obstructive Sleep Apnea: Is Salvation Just Around a Corner?Am J Respir Crit Care Med. 2019 May 15;199(10):1186-1187. doi: 10.1164/rccm.201811-2135ED. Am J Respir Crit Care Med. 2019. PMID: 30521761 Free PMC article. No abstract available.
-
Designer Receptors Exclusively Activated by Designer Drugs Approach to Treatment of Sleep-disordered Breathing.Am J Respir Crit Care Med. 2021 Jan 1;203(1):102-110. doi: 10.1164/rccm.202002-0321OC. Am J Respir Crit Care Med. 2021. PMID: 32673075 Free PMC article.
-
Neurostimulation Treatment of OSA.Chest. 2018 Dec;154(6):1435-1447. doi: 10.1016/j.chest.2018.08.1070. Epub 2018 Sep 14. Chest. 2018. PMID: 30222959 Free PMC article. Review.
-
Optical and pharmacological manipulation of hypoglossal motor nucleus identifies differential effects of taltirelin on sleeping tonic motor activity and responsiveness.Sci Rep. 2023 Jul 29;13(1):12299. doi: 10.1038/s41598-023-39562-z. Sci Rep. 2023. PMID: 37516800 Free PMC article.
-
Carotid Bodies and the Integrated Cardiorespiratory Response to Hypoxia.Physiology (Bethesda). 2018 Jul 1;33(4):281-297. doi: 10.1152/physiol.00014.2018. Physiology (Bethesda). 2018. PMID: 29897299 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

