α-Synuclein's Uniquely Long Amphipathic Helix Enhances its Membrane Binding and Remodeling Capacity
- PMID: 28239748
- PMCID: PMC5394797
- DOI: 10.1007/s00232-017-9946-1
α-Synuclein's Uniquely Long Amphipathic Helix Enhances its Membrane Binding and Remodeling Capacity
Erratum in
-
Correction to: α-Synuclein's Uniquely Long Amphipathic Helix Enhances its Membrane Binding and Remodeling Capacity.J Membr Biol. 2018 Dec;251(5-6):757. doi: 10.1007/s00232-018-0042-y. J Membr Biol. 2018. PMID: 30054671 Free PMC article.
Abstract
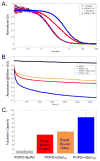
α-Synuclein is the primary protein found in Lewy bodies, the protein and lipid aggregates associated with Parkinson's disease and Lewy body dementia. The protein folds into a uniquely long amphipathic α-helix (AH) when bound to a membrane, and at high enough concentrations, it induces large-scale remodeling of membranes (tubulation and vesiculation). By engineering a less hydrophobic variant of α-Synuclein, we previously showed that the energy associated with binding of α-Synuclein's AH correlates with the extent of membrane remodeling (Braun et al. in J Am Chem Soc 136:9962-9972, 2014). In this study, we combine fluorescence correlation spectroscopy, electron microscopy, and vesicle clearance assays with coarse-grained molecular dynamics simulations to test the impact of decreasing the length of the amphipathic helix on membrane binding energy and tubulation. We show that truncation of α-Synuclein's AH length by approximately 15% reduces both its membrane binding affinity (by fivefold) and membrane remodeling capacity (by nearly 50% on per mole of bound protein basis). Results from simulations correlate well with the experiments and lend support to the idea that at high protein density there is a stabilization of individual, protein-induced membrane curvature fields. The extent to which these curvature fields are stabilized, a function of binding energy, dictates the extent of tubulation. Somewhat surprisingly, we find that this stabilization does not correlate directly with the geometric distribution of the proteins on the membrane surface.
Keywords: Alpha-Synuclein; Membrane remodeling; Tubulation.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

