Extended treatment for cigarette smoking cessation: a randomized control trial
- PMID: 28239942
- PMCID: PMC5503769
- DOI: 10.1111/add.13806
Extended treatment for cigarette smoking cessation: a randomized control trial
Abstract
Aim: To test the potential benefit of extending cognitive-behavioral therapy (CBT) relative to not extending CBT on long-term abstinence from smoking.
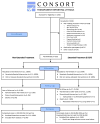
Design: Two-group parallel randomized controlled trial. Patients were randomized to receive non-extended CBT (n = 111) or extended CBT (n = 112) following a 26-week open-label treatment.
Setting: Community clinic in the United States.
Participants: A total of 219 smokers (mean age: 43 years; mean cigarettes/day: 18).
Intervention: All participants received 10 weeks of combined CBT + bupropion sustained release (bupropion SR) + nicotine patch and were continued on CBT and either no medications if abstinent, continued bupropion + nicotine replacement therapy (NRT) if increased craving or depression scores, or varenicline if still smoking at 10 weeks. Half the participants were randomized at 26 weeks to extended CBT (E-CBT) to week 48 and half to non-extended CBT (no additional CBT sessions).
Measurements: The primary outcome was expired CO-confirmed, 7-day point-prevalence (PP) at 52- and 104-week follow-up. Analyses were based on intention-to-treat.
Findings: PP abstinence rates at the 52-week follow-up were comparable across non-extended CBT (40%) and E-CBT (39%) groups [odds ratio (OR) = 0.99; 95% confidence interval (CI) = 0.55, 1.78]. A similar pattern was observed across non-extended CBT (39%) and E-CBT (33%) groups at the 104-week follow-up (OR = 0.79; 95% CI= 0.44, 1.40).
Conclusion: Prolonging cognitive-behavioral therapy from 26 to 48 weeks does not appear to improve long-term abstinence from smoking.
Keywords: Abstinence; adaptive treatment; bupropion SR; clinical trial; cognitive behavioral therapy; extended treatment; nicotine dependence; nicotine replacement therapy smoking cessation; smoking cessation; varenicline.
© 2017 Society for the Study of Addiction.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Comment in
-
Commentary on Laude et al. (2017): Extended treatment for cigarette smoking cessation.Addiction. 2017 Aug;112(8):1460-1461. doi: 10.1111/add.13884. Addiction. 2017. PMID: 28691271 No abstract available.
References
-
- Smith AL, Chapman S. Quitting smoking unassisted: the 50-year research neglect of a major public health phenomenon. JAMA. 2014;311:137–138. - PubMed
-
- Cahill K, Stevens S, Lancaster T. Pharmacological treatments for smoking cessation. JAMA. 2014;311:193–194. - PubMed
-
- Volkow ND, Morales M. The Brain on Drugs: From Reward to Addiction. Cell. 2015;162:712–725. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous