General Characteristics and Cytotoxic Effects of Nano-Poly (Butyl Cyanoacrylate) Containing Carboplatin on Ovarian Cancer Cells
- PMID: 28240014
- PMCID: PMC5563124
- DOI: 10.22034/APJCP.2017.18.1.87
General Characteristics and Cytotoxic Effects of Nano-Poly (Butyl Cyanoacrylate) Containing Carboplatin on Ovarian Cancer Cells
Abstract
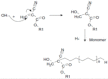
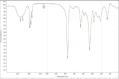
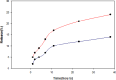
The initial response to treatment and subsequent development of resistance to carboplatin are very important challenges. Use of nano drug delivery is a new method to replace standard chemotherapy. In this research, both non-PEGylated and PEGylated nanoparticles (NPs) were prepared by mini-emulsion polymerization of poly (butyl cyanoacrylate) (PBCA) NPs. Characteristics such as size, polydispersity index (PDI), zeta potential, drug release, and stability were examined. In addition, infrared spectroscopy was used for description of the produced NPs. Then, cytotoxicity effects of both formulations were studied on the A2780CIS ovarian cancer cell line with incubation for 24, 48, and 72h. Examination of characteristics of loaded carboplatin on the PBCA NPs under suitable laboratory conditions showed a positive effect of PEG on their properties. Cytotoxicity studies demonstrated greater toxicity with both formulations of nano-drugs than the free drug. The results indicated that PBCA NPs can be considered as suitable candidates for nano-drugs in chemotherapy.
Keywords: Ovarian cancer; carboplatin; poly (butyl Cyanoacrylate) nanoparticle; nano-drug delivery.
Creative Commons Attribution License
Figures



Similar articles
-
Reversion of multidrug resistance by co-encapsulation of doxorubicin and curcumin in chitosan/poly(butyl cyanoacrylate) nanoparticles.Int J Pharm. 2012 Apr 15;426(1-2):193-201. doi: 10.1016/j.ijpharm.2012.01.020. Epub 2012 Jan 17. Int J Pharm. 2012. PMID: 22274587
-
Box-Behnken optimization design and enhanced oral bioavailability of thymopentin-loaded poly (butyl cyanoacrylate) nanoparticles.Pharmazie. 2011 May;66(5):339-47. Pharmazie. 2011. PMID: 21699067
-
In vitro and in vivo evaluation of 10-hydroxycamptothecin-loaded poly (n-butyl cyanoacrylate) nanoparticles prepared by miniemulsion polymerization.Colloids Surf B Biointerfaces. 2018 Feb 1;162:25-34. doi: 10.1016/j.colsurfb.2017.11.029. Epub 2017 Nov 12. Colloids Surf B Biointerfaces. 2018. PMID: 29145001
-
Targeting Leishmania amazonensis amastigotes through macrophage internalisation of a hydroxymethylnitrofurazone nanostructured polymeric system.Int J Antimicrob Agents. 2017 Jul;50(1):88-92. doi: 10.1016/j.ijantimicag.2017.01.033. Epub 2017 Apr 26. Int J Antimicrob Agents. 2017. PMID: 28454918
-
Quinoline-n-butylcyanoacrylate-based nanoparticles for brain targeting for the diagnosis of Alzheimer's disease.Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2010 Jan-Feb;2(1):35-47. doi: 10.1002/wnan.59. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2010. PMID: 20049829 Review.
Cited by
-
Smart Polymeric Nanoparticles in Cancer Immunotherapy.Pharmaceutics. 2023 Feb 26;15(3):775. doi: 10.3390/pharmaceutics15030775. Pharmaceutics. 2023. PMID: 36986636 Free PMC article. Review.
-
Targeted Nanocarrier-Based Drug Delivery Strategies for Improving the Therapeutic Efficacy of PARP Inhibitors against Ovarian Cancer.Int J Mol Sci. 2024 Jul 30;25(15):8304. doi: 10.3390/ijms25158304. Int J Mol Sci. 2024. PMID: 39125873 Free PMC article. Review.
-
Anticancer Effect of Cisplatin-Loaded Poly (Butylcyanoacrylate) Nanoparticles on A172 Brain Cancer Cells Line.Asian Pac J Cancer Prev. 2019 Jan 25;20(1):303-309. doi: 10.31557/APJCP.2019.20.1.303. Asian Pac J Cancer Prev. 2019. PMID: 30678454 Free PMC article.
-
Caffeine-folic acid-loaded-chitosan nanoparticles combined with methotrexate as a novel HepG2 immunotherapy targeting adenosine A2A receptor downstream cascade.BMC Complement Med Ther. 2023 Oct 27;23(1):384. doi: 10.1186/s12906-023-04212-4. BMC Complement Med Ther. 2023. PMID: 37891562 Free PMC article.
-
The Sources of Essential Fatty Acids for Allergic and Cancer Patients; a Connection with Insight into Mammalian Target of Rapamycin: A Narrative Review.Asian Pac J Cancer Prev. 2018 Sep 26;19(9):2391-2401. doi: 10.22034/APJCP.2018.19.9.2391. Asian Pac J Cancer Prev. 2018. PMID: 30255691 Free PMC article. Review.
References
-
- Andrieux K, Couvreur P. Polyalkylcyanoacrylate nanoparticles for delivery of drugs across the blood–brain barrier. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2009;1:463–463. - PubMed
-
- Antonietti M, Landfester K. Polyreactions in miniemulsions. Prog. Polym Sci. 2002;27:689–689.
-
- Behan N, Birkinshaw C, Clarke N. Poly n-butyl cyanoacrylate nanoparticles:a mechanistic study of polymerisation and particle formation. Biomaterials. 2001;22:1335–1335. - PubMed
-
- Douglas S, Illum L, Davis S. Particle size and size distribution of poly (butyl 2-cyanoacrylate) nanoparticles. II. Influence of stabilizers. J Colloid Interface Sci. 1985;103:154–154.
-
- Douglas S, Illum L, Davis S, et al. Particle size and size distribution of poly (butyl-2-cyanoacrylate) nanoparticles:I. Influence of physicochemical factors. J Colloid Interface Sci. 1984;101:149–149.

