Mammalian TRAPPIII Complex positively modulates the recruitment of Sec13/31 onto COPII vesicles
- PMID: 28240221
- PMCID: PMC5327430
- DOI: 10.1038/srep43207
Mammalian TRAPPIII Complex positively modulates the recruitment of Sec13/31 onto COPII vesicles
Abstract
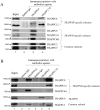
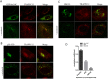
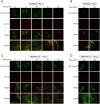
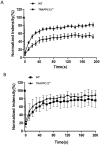
The Transport protein particle (TRAPP) complex is a tethering factor for COPII vesicle. Of three forms of TRAPP (TRAPPI, II and III) complexes identified so far, TRAPPIII has been largely considered to play a role in autophagy. While depletion of TRAPPIII specific subunits caused defects in the early secretory pathway and TRAPPIII might interact with components of the COPII vesicle coat, its exact role remains to be determined. In this study, we studied the function of TRAPPIII in early secretory pathway using a TRAPPIII-specific subunit, TRAPPC12, as starting point. We found that TRAPPC12 was localized to the ER exit sites and ERGIC. In cells deleted with TRAPPC12, ERGIC and to a lesser extent, the Golgi became dispersed. ER-to-Golgi transport was also delayed. TRAPPC12, but not TRAPPC8, bound to Sec13/Sec31A tetramer but each Sec protein alone could not interact with TRAPPC12. TRAPPIII positively modulated the assembly of COPII outer layer during COPII vesicle formation. These results identified a novel function of TRAPPIII as a positive modulator of the outer layer of the COPII coat.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



References
-
- Budnik A. & Stephens D. J. ER exit sites–localization and control of COPII vesicle formation. FEBS letters 583, 3796–3803 (2009). - PubMed
-
- Sacher M. et al. TRAPP I implicated in the specificity of tethering in ER-to-Golgi transport. Mol Cell 7, 433–442 (2001). - PubMed
-
- Cai H. et al. TRAPPI tethers COPII vesicles by binding the coat subunit Sec23. Nature 445, 941–944 (2007). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

