Interleukin-27 Enhances the Potential of Reactive Oxygen Species Generation from Monocyte-derived Macrophages and Dendritic cells by Induction of p47phox
- PMID: 28240310
- PMCID: PMC5327488
- DOI: 10.1038/srep43441
Interleukin-27 Enhances the Potential of Reactive Oxygen Species Generation from Monocyte-derived Macrophages and Dendritic cells by Induction of p47phox
Abstract
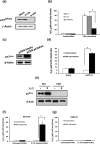
Interleukin (IL)-27, a member of the IL-12 cytokine family, plays an important and diverse role in the function of the immune system. We have previously demonstrated that IL-27 is an anti-viral cytokine which inhibits HIV-1, HIV-2, Influenza virus and herpes simplex virus infection, and enhances the potential of reactive oxygen species (ROS) generating activity during differentiation of monocytes to macrophages. In this study, we further investigated the mechanism of the enhanced potential for ROS generation by IL-27. Real time PCR, western blot and knock down assays demonstrate that IL-27 is able to enhance the potential of superoxide production not only during differentiation but also in terminally differentiated-macrophages and immature dendritic cells (iDC) in association with the induction of p47phox, a cytosolic component of the ROS producing enzyme, NADPH oxidase, and the increase in amounts of phosphorylated p47phox upon stimulation. We also demonstrate that IL-27 is able to induce extracellular superoxide dismutase during differentiation of monocytes but not in terminal differentiated macrophages. Since ROS plays an important role in a variety of inflammation, our data demonstrate that IL-27 is a potent regulator of ROS induction and may be a novel therapeutic target.
Conflict of interest statement
The authors declare no competing financial interests.
Figures






Similar articles
-
Cellular Differentiation of Human Monocytes Is Regulated by Time-Dependent Interleukin-4 Signaling and the Transcriptional Regulator NCOR2.Immunity. 2017 Dec 19;47(6):1051-1066.e12. doi: 10.1016/j.immuni.2017.11.024. Immunity. 2017. PMID: 29262348 Free PMC article.
-
IL-6 switches the differentiation of monocytes from dendritic cells to macrophages.Nat Immunol. 2000 Dec;1(6):510-4. doi: 10.1038/82763. Nat Immunol. 2000. PMID: 11101873
-
Generation of CD1+RelB+ dendritic cells and tartrate-resistant acid phosphatase-positive osteoclast-like multinucleated giant cells from human monocytes.Blood. 1996 Nov 15;88(10):4029-39. Blood. 1996. PMID: 8916970
-
Activities of granulocyte-macrophage colony-stimulating factor and interleukin-3 on monocytes.Am J Hematol. 2004 Apr;75(4):179-89. doi: 10.1002/ajh.20010. Am J Hematol. 2004. PMID: 15054806
-
p47(phox) directs murine macrophage cell fate decisions.Am J Pathol. 2012 Mar;180(3):1049-1058. doi: 10.1016/j.ajpath.2011.11.019. Epub 2012 Jan 2. Am J Pathol. 2012. PMID: 22222227 Free PMC article.
Cited by
-
IL-27 posttranslationally regulates Y-box binding protein-1 to inhibit HIV-1 replication in human CD4+ T cells.AIDS. 2019 Oct 1;33(12):1819-1830. doi: 10.1097/QAD.0000000000002288. AIDS. 2019. PMID: 31274540 Free PMC article.
-
ROS systems are a new integrated network for sensing homeostasis and alarming stresses in organelle metabolic processes.Redox Biol. 2020 Oct;37:101696. doi: 10.1016/j.redox.2020.101696. Epub 2020 Aug 27. Redox Biol. 2020. PMID: 32950427 Free PMC article. Review.
-
Exploring the role of interleukin-27 as a regulator of neuronal survival in central nervous system diseases.Neural Regen Res. 2022 Oct;17(10):2149-2152. doi: 10.4103/1673-5374.336134. Neural Regen Res. 2022. PMID: 35259821 Free PMC article. Review.
-
A novel microRNA, hsa-miR-6852 differentially regulated by Interleukin-27 induces necrosis in cervical cancer cells by downregulating the FoxM1 expression.Sci Rep. 2018 Jan 17;8(1):900. doi: 10.1038/s41598-018-19259-4. Sci Rep. 2018. PMID: 29343703 Free PMC article.
-
Interleukin-27 and Its Diverse Effects on Bacterial Infections.Front Immunol. 2021 May 17;12:678515. doi: 10.3389/fimmu.2021.678515. eCollection 2021. Front Immunol. 2021. PMID: 34079555 Free PMC article. Review.
References
-
- Pflanz S. et al.. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4(+) T cells. Immunity 16, 779–790, doi: S1074761302003242 (2002). - PubMed
-
- Villarino A. V., Huang E. & Hunter C. A. Understanding the pro- and anti-inflammatory properties of IL-27. Journal of immunology 173, 715–720 (2004). - PubMed
-
- Pflanz S. et al.. WSX-1 and glycoprotein 130 constitute a signal-transducing receptor for IL-27. Journal of immunology 172, 2225–2231 (2004). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

