Autocrine lysophosphatidic acid signaling activates β-catenin and promotes lung allograft fibrosis
- PMID: 28240604
- PMCID: PMC5373892
- DOI: 10.1172/JCI88896
Autocrine lysophosphatidic acid signaling activates β-catenin and promotes lung allograft fibrosis
Abstract
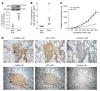
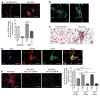
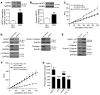
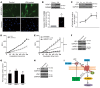
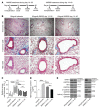
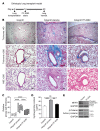
Tissue fibrosis is the primary cause of long-term graft failure after organ transplantation. In lung allografts, progressive terminal airway fibrosis leads to an irreversible decline in lung function termed bronchiolitis obliterans syndrome (BOS). Here, we have identified an autocrine pathway linking nuclear factor of activated T cells 2 (NFAT1), autotaxin (ATX), lysophosphatidic acid (LPA), and β-catenin that contributes to progression of fibrosis in lung allografts. Mesenchymal cells (MCs) derived from fibrotic lung allografts (BOS MCs) demonstrated constitutive nuclear β-catenin expression that was dependent on autocrine ATX secretion and LPA signaling. We found that NFAT1 upstream of ATX regulated expression of ATX as well as β-catenin. Silencing NFAT1 in BOS MCs suppressed ATX expression, and sustained overexpression of NFAT1 increased ATX expression and activity in non-fibrotic MCs. LPA signaling induced NFAT1 nuclear translocation, suggesting that autocrine LPA synthesis promotes NFAT1 transcriptional activation and ATX secretion in a positive feedback loop. In an in vivo mouse orthotopic lung transplant model of BOS, antagonism of the LPA receptor (LPA1) or ATX inhibition decreased allograft fibrosis and was associated with lower active β-catenin and dephosphorylated NFAT1 expression. Lung allografts from β-catenin reporter mice demonstrated reduced β-catenin transcriptional activation in the presence of LPA1 antagonist, confirming an in vivo role for LPA signaling in β-catenin activation.
Conflict of interest statement
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

