Randomized Clinical Trial of a Combination of an Inhaled Corticosteroid and Beta Agonist in Patients at Risk of Developing the Acute Respiratory Distress Syndrome
- PMID: 28240689
- PMCID: PMC5392150
- DOI: 10.1097/CCM.0000000000002284
Randomized Clinical Trial of a Combination of an Inhaled Corticosteroid and Beta Agonist in Patients at Risk of Developing the Acute Respiratory Distress Syndrome
Abstract
Objectives: Effective pharmacologic treatments directly targeting lung injury in patients with the acute respiratory distress syndrome are lacking. Early treatment with inhaled corticosteroids and beta agonists may reduce progression to acute respiratory distress syndrome by reducing lung inflammation and enhancing alveolar fluid clearance.
Design: Double-blind, randomized clinical trial (ClinicalTrials.gov: NCT01783821). The primary outcome was longitudinal change in oxygen saturation divided by the FIO2 (S/F) through day 5. We also analyzed categorical change in S/F by greater than 20%. Other outcomes included need for mechanical ventilation and development of acute respiratory distress syndrome.
Setting: Five academic centers in the United States.
Patients: Adult patients admitted through the emergency department at risk for acute respiratory distress syndrome.
Interventions: Aerosolized budesonide/formoterol versus placebo bid for up to 5 days.
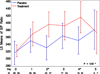
Measurements and main results: Sixty-one patients were enrolled from September 3, 2013, to June 9, 2015. Median time from presentation to first study drug was less than 9 hours. More patients in the control group had shock at enrollment (14 vs 3 patients). The longitudinal increase in S/F was greater in the treatment group (p = 0.02) and independent of shock (p = 0.04). Categorical change in S/F improved (p = 0.01) but not after adjustment for shock (p = 0.15). More patients in the placebo group developed acute respiratory distress syndrome (7 vs 0) and required mechanical ventilation (53% vs 21%).
Conclusions: Early treatment with inhaled budesonide/formoterol in patients at risk for acute respiratory distress syndrome is feasible and improved oxygenation as assessed by S/F. These results support further study to test the efficacy of inhaled corticosteroids and beta agonists for prevention of acute respiratory distress syndrome.
Conflict of interest statement
Figures


Comment in
-
Steroids and β-Agonists in Acute Respiratory Distress Syndrome: Timing Is Everything.Crit Care Med. 2017 May;45(5):914-915. doi: 10.1097/CCM.0000000000002385. Crit Care Med. 2017. PMID: 28410310 No abstract available.
References
-
- Ashbaugh DG, Bigelow DB, Petty TL, et al. Acute respiratory distress in adults. Lancet. 1967;2:319–323. - PubMed
-
- Villar J, Blanco J, Anon JM, et al. ALIEN Network. The ALIEN study: incidence and outcome of acute respiratory distress syndrome in the era of lung protective ventilation. Intensive Care Med. 2011;37:1932–1941. - PubMed
-
- Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301–1308. - PubMed
-
- National Heart Lung Blood Institute Acute Respiratory Distress Syndrome Clinical Trials Network. Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354:2564–2575. - PubMed
-
- Papazian L, Forel J-M, Gacouin A, et al. ACURASYS Study Investigators. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010;363:1107–1116. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

