Gaze-informed, task-situated representation of space in primate hippocampus during virtual navigation
- PMID: 28241007
- PMCID: PMC5328243
- DOI: 10.1371/journal.pbio.2001045
Gaze-informed, task-situated representation of space in primate hippocampus during virtual navigation
Abstract
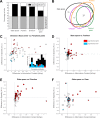
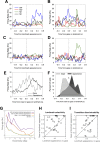
To elucidate how gaze informs the construction of mental space during wayfinding in visual species like primates, we jointly examined navigation behavior, visual exploration, and hippocampal activity as macaque monkeys searched a virtual reality maze for a reward. Cells sensitive to place also responded to one or more variables like head direction, point of gaze, or task context. Many cells fired at the sight (and in anticipation) of a single landmark in a viewpoint- or task-dependent manner, simultaneously encoding the animal's logical situation within a set of actions leading to the goal. Overall, hippocampal activity was best fit by a fine-grained state space comprising current position, view, and action contexts. Our findings indicate that counterparts of rodent place cells in primates embody multidimensional, task-situated knowledge pertaining to the target of gaze, therein supporting self-awareness in the construction of space.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
-
- O’Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;34: 171–175. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

