Cytokines and microbicidal molecules regulated by IL-32 in THP-1-derived human macrophages infected with New World Leishmania species
- PMID: 28241012
- PMCID: PMC5344527
- DOI: 10.1371/journal.pntd.0005413
Cytokines and microbicidal molecules regulated by IL-32 in THP-1-derived human macrophages infected with New World Leishmania species
Abstract
Background: Interleukin-32 (IL-32) is expressed in lesions of patients with American Tegumentary Leishmaniasis (ATL), but its precise role in the disease remains unknown.
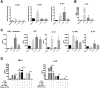
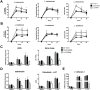
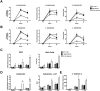
Methodology/principal findings: In the present study, silencing and overexpression of IL-32 was performed in THP-1-derived macrophages infected with Leishmania (Viannia) braziliensis or L. (Leishmania) amazonensis to investigate the role of IL-32 in infection. We report that Leishmania species induces IL-32γ, and show that intracellular IL-32γ protein production is dependent on endogenous TNFα. Silencing or overexpression of IL-32 demonstrated that this cytokine is closely related to TNFα and IL-8. Remarkably, the infection index was augmented in the absence of IL-32 and decreased in cells overexpressing this cytokine. Mechanistically, these effects can be explained by nitric oxide cathelicidin and β-defensin 2 production regulated by IL-32.
Conclusions: Thus, endogenous IL-32 is a crucial cytokine involved in the host defense against Leishmania parasites.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
IL-32γ promotes the healing of murine cutaneous lesions caused by Leishmania braziliensis infection in contrast to Leishmania amazonensis.Parasit Vectors. 2017 Jul 14;10(1):336. doi: 10.1186/s13071-017-2268-4. Parasit Vectors. 2017. PMID: 28709468 Free PMC article.
-
Interleukin 32γ (IL-32γ) is highly expressed in cutaneous and mucosal lesions of American Tegumentary Leishmaniasis patients: association with tumor necrosis factor (TNF) and IL-10.BMC Infect Dis. 2014 May 9;14:249. doi: 10.1186/1471-2334-14-249. BMC Infect Dis. 2014. PMID: 24884781 Free PMC article.
-
IL-15 enhances the capacity of primary human macrophages to control Leishmania braziliensis infection by IL-32/vitamin D dependent and independent pathways.Parasitol Int. 2020 Jun;76:102097. doi: 10.1016/j.parint.2020.102097. Epub 2020 Feb 27. Parasitol Int. 2020. PMID: 32114085
-
A Critical Overview of Interleukin 32 in Leishmaniases.Front Immunol. 2022 Mar 3;13:849340. doi: 10.3389/fimmu.2022.849340. eCollection 2022. Front Immunol. 2022. PMID: 35309341 Free PMC article. Review.
-
Interleukin-27 Functional Duality Balances Leishmania Infectivity and Pathogenesis.Front Immunol. 2020 Aug 7;11:1573. doi: 10.3389/fimmu.2020.01573. eCollection 2020. Front Immunol. 2020. PMID: 32849534 Free PMC article. Review.
Cited by
-
Cathelicidin Contributes to the Restriction of Leishmania in Human Host Macrophages.Front Immunol. 2019 Nov 22;10:2697. doi: 10.3389/fimmu.2019.02697. eCollection 2019. Front Immunol. 2019. PMID: 31824492 Free PMC article.
-
Gold(I) and Silver(I) Complexes Containing Hybrid Sulfonamide/Thiourea Ligands as Potential Leishmanicidal Agents.Pharmaceutics. 2024 Mar 25;16(4):452. doi: 10.3390/pharmaceutics16040452. Pharmaceutics. 2024. PMID: 38675113 Free PMC article.
-
Trained immunity: a cutting edge approach for designing novel vaccines against parasitic diseases?Front Immunol. 2023 Oct 6;14:1252554. doi: 10.3389/fimmu.2023.1252554. eCollection 2023. Front Immunol. 2023. PMID: 37868995 Free PMC article. Review.
-
Vitamin d and leishmaniasis: Neither seasonal nor risk factor in canine host but potential adjuvant treatment through cbd103 expression.PLoS Negl Trop Dis. 2021 Aug 16;15(8):e0009681. doi: 10.1371/journal.pntd.0009681. eCollection 2021 Aug. PLoS Negl Trop Dis. 2021. PMID: 34398874 Free PMC article.
-
Cytokines: Key Determinants of Resistance or Disease Progression in Visceral Leishmaniasis: Opportunities for Novel Diagnostics and Immunotherapy.Front Immunol. 2019 Apr 5;10:670. doi: 10.3389/fimmu.2019.00670. eCollection 2019. Front Immunol. 2019. PMID: 31024534 Free PMC article. Review.
References
-
- Heinhuis B, Netea MG, van den Berg WB, Dinarello CA., Joosten LAB. Interleukin-32: A predominantly intracellular proinflammatory mediator that controls cell activation and cell death. Cytokine. Elsevier Ltd; 2012;60: 321–327. - PubMed
-
- Kim S-H, Han S-Y, Azam T, Yoon D-Y, Dinarello CA. Interleukin-32. Immunity. 2005;22: 131–142. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

