Benefit of adjuvant immunotherapy in renal cell carcinoma: A myth or a reality?
- PMID: 28241027
- PMCID: PMC5328261
- DOI: 10.1371/journal.pone.0172341
Benefit of adjuvant immunotherapy in renal cell carcinoma: A myth or a reality?
Abstract
Background: The benefit of adjuvant immunotherapy after nephrectomy in renal cell carcinoma (RCC) is controversial. The present study aimed to examine the possible benefit of adjuvant immunotherapy in various clinical settings.
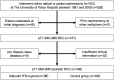
Methods: We retrospectively reviewed 436 patients with pT1-3N0-2M0 RCC who underwent radical or partial nephrectomy with curative intent at our institution between 1981 and 2009. Of them, 98 (22.5%) patients received adjuvant interferon-α (IFN-α) after surgery (adjuvant IFN-α group), while 338 (77.5%) did not (control group). The primary endpoint was cancer-specific survival (CSS). Univariate and multivariate analyses were conducted using log-rank tests and Cox proportional hazards models, respectively.
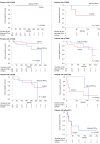
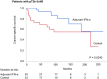
Results: Fifty-two (11.9%) patients died from RCC with a median follow-up period of 96 months. Preliminary univariate analyses comparing CSS among treatment groups in each TNM setting revealed that CSS in the control group was equal or superior to that in the adjuvant IFN-α group in earlier stages, while the opposite trend was observed in more advanced stages. We evaluated the TNM cutoffs and demonstrated maximized benefit of adjuvant IFN-α in patients with pT2b-3cN0 (P = 0.0240). In multivariate analysis, ≥pT3 and pN1-2 were independent predictors for poor CSS in all patients. In the subgroups with ≥pT2 disease (n = 123), pN1-2 and no adjuvant treatment were significant poor prognostic factors.
Conclusions: Adjuvant immunotherapy after nephrectomy may be beneficial in pT2b-3cN0 RCC. Careful consideration is, however, required for interpretation of this observational study because of its selection bias and adverse effects of IFN-α.
Conflict of interest statement
Figures
References
-
- Motzer RJ, Jonasch E, Agarwal N, Beard C, Bhayani S, Bolger GB, et al. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Kidney Cancer Version 2.2014. Fort Washington: National Comprehensive Cancer Network, Inc; 2014.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

