Age-Related Loss in Bone Mineral Density of Rats Fed Lifelong on a Fish Oil-Based Diet Is Avoided by Coenzyme Q10 Addition
- PMID: 28241421
- PMCID: PMC5331607
- DOI: 10.3390/nu9020176
Age-Related Loss in Bone Mineral Density of Rats Fed Lifelong on a Fish Oil-Based Diet Is Avoided by Coenzyme Q10 Addition
Abstract
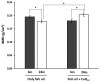
During aging, bone mass declines increasing osteoporosis and fracture risks. Oxidative stress has been related to this bone loss, making dietary compounds with antioxidant properties a promising weapon. Male Wistar rats were maintained for 6 or 24 months on diets with fish oil as unique fat source, supplemented or not with coenzyme Q10 (CoQ10), to evaluate the potential of adding this molecule to the n-3 polyunsaturated fatty acid (n-3 PUFA)-based diet for bone mineral density (BMD) preservation. BMD was evaluated in the femur. Serum osteocalcin, osteopontin, receptor activator of nuclear factor-κB ligand, ostroprotegerin, parathyroid hormone, urinary F₂-isoprostanes, and lymphocytes DNA strand breaks were also measured. BMD was lower in aged rats fed a diet without CoQ10 respect than their younger counterparts, whereas older animals receiving CoQ10 showed the highest BMD. F₂-isoprostanes and DNA strand breaks showed that oxidative stress was higher during aging. Supplementation with CoQ10 prevented oxidative damage to lipid and DNA, in young and old animals, respectively. Reduced oxidative stress associated to CoQ10 supplementation of this n-3 PUFA-rich diet might explain the higher BMD found in aged rats in this group of animals.
Keywords: antioxidants; dietary fat; n-3 PUFA; oxidative stress; ubiquinone.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Loss of Bone Mineral Density Associated with Age in Male Rats Fed on Sunflower Oil Is Avoided by Virgin Olive Oil Intake or Coenzyme Q Supplementation.Int J Mol Sci. 2017 Jun 29;18(7):1397. doi: 10.3390/ijms18071397. Int J Mol Sci. 2017. PMID: 28661441 Free PMC article.
-
Enhanced anti-oxidant protection of liver membranes in long-lived rats fed on a coenzyme Q10-supplemented diet.Exp Gerontol. 2005 Aug-Sep;40(8-9):694-706. doi: 10.1016/j.exger.2005.07.003. Exp Gerontol. 2005. PMID: 16125350
-
Protective effect of supplementation of fish oil with high n-3 polyunsaturated fatty acids against oxidative stress-induced DNA damage of rat liver in vivo.J Agric Food Chem. 2003 Sep 24;51(20):6073-9. doi: 10.1021/jf030141v. J Agric Food Chem. 2003. PMID: 13129319
-
Coenzyme Q addition to an n-6 PUFA-rich diet resembles benefits on age-related mitochondrial DNA deletion and oxidative stress of a MUFA-rich diet in rat heart.Mech Ageing Dev. 2010 Jan;131(1):38-47. doi: 10.1016/j.mad.2009.11.004. Epub 2009 Dec 3. Mech Ageing Dev. 2010. PMID: 19948181
-
Coenzyme Q10 Supplementation Improves Adipokine Levels and Alleviates Inflammation and Lipid Peroxidation in Conditions of Metabolic Syndrome: A Meta-Analysis of Randomized Controlled Trials.Int J Mol Sci. 2020 May 4;21(9):3247. doi: 10.3390/ijms21093247. Int J Mol Sci. 2020. PMID: 32375340 Free PMC article. Review.
Cited by
-
Dietary ω-3 Fatty Acid Supplementation Improves Murine Sickle Cell Bone Disease and Reprograms Adipogenesis.Antioxidants (Basel). 2021 May 18;10(5):799. doi: 10.3390/antiox10050799. Antioxidants (Basel). 2021. PMID: 34070133 Free PMC article.
-
Genome-Protecting Compounds as Potential Geroprotectors.Int J Mol Sci. 2020 Jun 24;21(12):4484. doi: 10.3390/ijms21124484. Int J Mol Sci. 2020. PMID: 32599754 Free PMC article. Review.
-
Differential expression of circRNAs of testes with high and low sperm motility in Yili geese.Front Genet. 2022 Sep 26;13:970097. doi: 10.3389/fgene.2022.970097. eCollection 2022. Front Genet. 2022. PMID: 36226183 Free PMC article.
-
Modulation by hydroxytyrosol of oxidative stress and antitumor activities of paclitaxel in breast cancer.Eur J Nutr. 2019 Apr;58(3):1203-1211. doi: 10.1007/s00394-018-1638-9. Epub 2018 Feb 21. Eur J Nutr. 2019. PMID: 29468462
-
Crosstalk between Lipid Metabolism and Bone Homeostasis: Exploring Intricate Signaling Relationships.Research (Wash D C). 2024 Aug 20;7:0447. doi: 10.34133/research.0447. eCollection 2024. Research (Wash D C). 2024. PMID: 39165638 Free PMC article.
References
-
- Alvioli L.V., Lindsay R. The female osteoporotic syndrome(s) In: Alvioli L.V., Krane S.M., editors. Metabolic Bone Disease and Clinically Related Disorder. WB Saunders Company; Philadelphia, PA, USA: 1990. pp. 397–451.
-
- Szulc P., Seeman E., Duboeuf F., Sornay-Rendu E., Delmas P.D. Bone fragility: Failure of periosteal apposition to compensate for increased endocortical resorption in postmenopausal women. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2006;21:1856–1863. doi: 10.1359/jbmr.060904. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

