The Role of Carbohydrate Response Element Binding Protein in Intestinal and Hepatic Fructose Metabolism
- PMID: 28241431
- PMCID: PMC5331612
- DOI: 10.3390/nu9020181
The Role of Carbohydrate Response Element Binding Protein in Intestinal and Hepatic Fructose Metabolism
Abstract
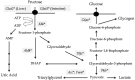
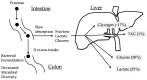
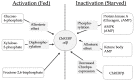
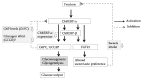
Many articles have discussed the relationship between fructose consumption and the incidence of obesity and related diseases. Fructose is absorbed in the intestine and metabolized in the liver to glucose, lactate, glycogen, and, to a lesser extent, lipids. Unabsorbed fructose causes bacterial fermentation, resulting in irritable bowl syndrome. Therefore, understanding the mechanisms underlying intestinal and hepatic fructose metabolism is important for the treatment of metabolic syndrome and fructose malabsorption. Carbohydrate response element binding protein (ChREBP) is a glucose-activated transcription factor that controls approximately 50% of de novo lipogenesis in the liver. ChREBP target genes are involved in glycolysis (Glut2, liver pyruvate kinase), fructolysis (Glut5, ketohexokinase), and lipogenesis (acetyl CoA carboxylase, fatty acid synthase). ChREBP gene deletion protects against high sucrose diet-induced and leptin-deficient obesity, because Chrebp-/- mice cannot consume fructose or sucrose. Moreover, ChREBP contributes to some of the physiological effects of fructose on sweet taste preference and glucose production through regulation of ChREBP target genes, such as fibroblast growth factor-21 and glucose-6-phosphatase catalytic subunits. Thus, ChREBP might play roles in fructose metabolism. Restriction of excess fructose intake will be beneficial for preventing not only metabolic syndrome but also irritable bowl syndrome.
Keywords: ChREBP; Glut5/SLC2A5; carbohydrate response element binding protein; fructolysis; fructose; glycolysis; ketohexokinase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
ChREBP deficiency leads to diarrhea-predominant irritable bowel syndrome.Metabolism. 2018 Aug;85:286-297. doi: 10.1016/j.metabol.2018.04.006. Epub 2018 Apr 15. Metabolism. 2018. PMID: 29669261 Free PMC article.
-
ChREBP: a glucose-activated transcription factor involved in the development of metabolic syndrome.Endocr J. 2008 Aug;55(4):617-24. doi: 10.1507/endocrj.k07e-110. Epub 2008 May 19. Endocr J. 2008. PMID: 18490833 Review.
-
Recent insights into the role of ChREBP in intestinal fructose absorption and metabolism.BMB Rep. 2018 Sep;51(9):429-436. doi: 10.5483/BMBRep.2018.51.9.197. BMB Rep. 2018. PMID: 30158026 Free PMC article. Review.
-
A critical role for ChREBP-mediated FGF21 secretion in hepatic fructose metabolism.Mol Metab. 2016 Nov 23;6(1):14-21. doi: 10.1016/j.molmet.2016.11.008. eCollection 2017 Jan. Mol Metab. 2016. PMID: 28123933 Free PMC article.
-
ChREBP regulates fructose-induced glucose production independently of insulin signaling.J Clin Invest. 2016 Nov 1;126(11):4372-4386. doi: 10.1172/JCI81993. Epub 2016 Sep 26. J Clin Invest. 2016. PMID: 27669460 Free PMC article.
Cited by
-
Thioredoxin interacting protein regulates age-associated neuroinflammation.Neurobiol Dis. 2021 Aug;156:105399. doi: 10.1016/j.nbd.2021.105399. Epub 2021 May 21. Neurobiol Dis. 2021. PMID: 34029695 Free PMC article.
-
Dietary fructose feeds hepatic lipogenesis via microbiota-derived acetate.Nature. 2020 Mar;579(7800):586-591. doi: 10.1038/s41586-020-2101-7. Epub 2020 Mar 18. Nature. 2020. PMID: 32214246 Free PMC article.
-
The Role of Fructose in Non-Alcoholic Steatohepatitis: Old Relationship and New Insights.Nutrients. 2021 Apr 16;13(4):1314. doi: 10.3390/nu13041314. Nutrients. 2021. PMID: 33923525 Free PMC article. Review.
-
A mitochondria-targeted fatty acid analogue influences hepatic glucose metabolism and reduces the plasma insulin/glucose ratio in male Wistar rats.PLoS One. 2019 Sep 24;14(9):e0222558. doi: 10.1371/journal.pone.0222558. eCollection 2019. PLoS One. 2019. PMID: 31550253 Free PMC article.
-
Phenolics-Rich Extracts of Dietary Plants as Regulators of Fructose Uptake in Caco-2 Cells via GLUT5 Involvement.Molecules. 2021 Aug 5;26(16):4745. doi: 10.3390/molecules26164745. Molecules. 2021. PMID: 34443333 Free PMC article.
References
-
- Elliott S.S., Keim N.L., Stern J.S., Teff K., Havel P.J. Fructose, weight gain, and the insulin resistance syndrome. Am. J. Clin. Nutr. 2002;76:911–922. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

