Quorum Sensing Inhibitors from the Sea Discovered Using Bacterial N-acyl-homoserine Lactone-Based Biosensors
- PMID: 28241461
- PMCID: PMC5367010
- DOI: 10.3390/md15030053
Quorum Sensing Inhibitors from the Sea Discovered Using Bacterial N-acyl-homoserine Lactone-Based Biosensors
Abstract
Marine natural products with antibiotic activity have been a rich source of drug discovery; however, the emergence of antibiotic-resistant bacterial strains has turned attention towards the discovery of alternative innovative strategies to combat pathogens. In many pathogenic bacteria, the expression of virulence factors is under the regulation of quorum sensing (QS). QS inhibitors (QSIs) present a promising alternative or potential synergistic treatment since they disrupt the signaling pathway used for intra- and interspecies coordination of expression of virulence factors. This review covers the set of molecules showing QSI activity that were isolated from marine organisms, including plants (algae), animals (sponges, cnidarians, and bryozoans), and microorganisms (bacteria, fungi, and cyanobacteria). The compounds found and the methods used for their isolation are the emphasis of this review.
Keywords: N-acyl homoserine lactones; antimicrobial resistance; marine natural products; quorum quenching; quorum-sensing inhibitors.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


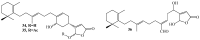

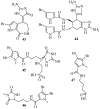
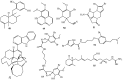

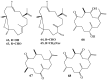
References
-
- Yong D., Toleman M.A., Giske C.G., Cho H.S., Sundman K., Lee K., Walsh T.R. Characterization of a new metallo-beta-lactamase gene, bla(NDM-1), and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob. Agents Chemother. 2009;53:5046–5054. doi: 10.1128/AAC.00774-09. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

