TREatment of ATopic eczema (TREAT) Registry Taskforce: protocol for an international Delphi exercise to identify a core set of domains and domain items for national atopic eczema registries
- PMID: 28241851
- PMCID: PMC5330088
- DOI: 10.1186/s13063-016-1765-7
TREatment of ATopic eczema (TREAT) Registry Taskforce: protocol for an international Delphi exercise to identify a core set of domains and domain items for national atopic eczema registries
Abstract
Background: Patients with moderate-to-severe atopic eczema (AE) often require photo- or systemic immunomodulatory therapies to induce disease remission and maintain long-term control. The current evidence to guide clinical management is small, despite the frequent and often off-label use of these treatments. Registries of patients on photo- and systemic immunomodulatory therapies could fill this gap, and the collection of a core set concerning these therapies in AE will allow direct comparisons across registries as well as data sharing and pooling. Using an eDelphi approach, the international TREatment of ATopic eczema (TREAT) Registry Taskforce aims to seek consensus between key stakeholders internationally on a core set of domains and domain items for AE patient registries with a research focus that collect data of children and adults on photo- and systemic immunomodulatory therapies.
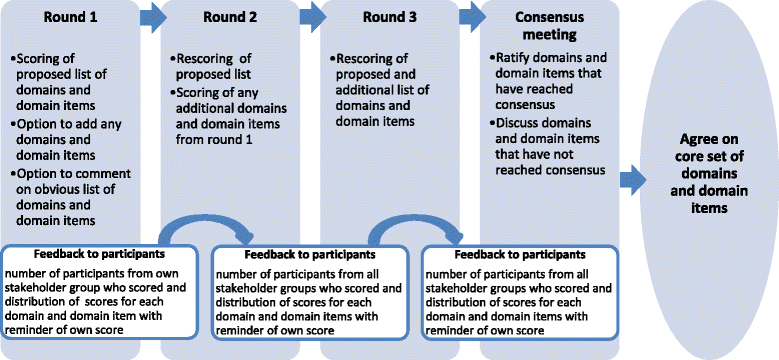
Methods/design: Participants from six stakeholder groups will be invited: doctors, nurses, non-clinical researchers, patients, as well as industry and regulatory body representatives. The eDelphi will comprise three sequential online rounds, requesting participants to rate the importance of each proposed domain and domain items. Participants will be able to add domains and domain items to the proposed list in round 1. A final consensus meeting will be held with representatives of each stakeholder group.
Discussion: Identifying a uniform core set of domains and domain items to be captured by AE patient registries will increase the utility of individual registries, and provide greater insight into the effectiveness, safety and cost-effectiveness of photo- and systemic immunomodulatory therapies to guide clinical management across dermatology centres and country borders.
Trial registration: Not applicable. This eDelphi study was registered in the Core Outcome Measures for Effectiveness Trials (COMET) database.
Keywords: Atopic dermatitis; Atopic eczema; Consensus methods; Core set; Daily practice data; Delphi; Disease registries; Immunomodulatory therapies; Interoperability; Patient registries.
Figures
Similar articles
-
TREatment of ATopic eczema (TREAT) Registry Taskforce: consensus on how and when to measure the core dataset for atopic eczema treatment research registries.Br J Dermatol. 2019 Sep;181(3):492-504. doi: 10.1111/bjd.17715. Epub 2019 Jun 23. Br J Dermatol. 2019. PMID: 30719709 Free PMC article.
-
TREatment of ATopic eczema (TREAT) Registry Taskforce: an international Delphi exercise to identify a core set of domains and domain items for national atopic eczema photo- and systemic therapy registries.Br J Dermatol. 2019 Apr;180(4):790-801. doi: 10.1111/bjd.16714. Epub 2018 Aug 3. Br J Dermatol. 2019. PMID: 29761486 Free PMC article.
-
A Global eDelphi Exercise to Identify Core Domains and Domain Items for the Development of a Global Registry of Alopecia Areata Disease Severity and Treatment Safety (GRASS).JAMA Dermatol. 2021 Apr 1;157(4):1-11. doi: 10.1001/jamadermatol.2020.5839. JAMA Dermatol. 2021. PMID: 33656556
-
The Harmonising Outcome Measures for Eczema (HOME) statement to assess clinical signs of atopic eczema in trials.J Allergy Clin Immunol. 2014 Oct;134(4):800-7. doi: 10.1016/j.jaci.2014.07.043. J Allergy Clin Immunol. 2014. PMID: 25282560 Review.
-
Identifying a Core Domain Set to Assess Psoriasis in Clinical Trials.JAMA Dermatol. 2018 Oct 1;154(10):1137-1144. doi: 10.1001/jamadermatol.2018.1165. JAMA Dermatol. 2018. PMID: 29874367 Free PMC article.
Cited by
-
TREatment of ATopic eczema (TREAT) Registry Taskforce: consensus on how and when to measure the core dataset for atopic eczema treatment research registries.Br J Dermatol. 2019 Sep;181(3):492-504. doi: 10.1111/bjd.17715. Epub 2019 Jun 23. Br J Dermatol. 2019. PMID: 30719709 Free PMC article.
-
TREatment of ATopic eczema (TREAT) Registry Taskforce: an international Delphi exercise to identify a core set of domains and domain items for national atopic eczema photo- and systemic therapy registries.Br J Dermatol. 2019 Apr;180(4):790-801. doi: 10.1111/bjd.16714. Epub 2018 Aug 3. Br J Dermatol. 2019. PMID: 29761486 Free PMC article.
-
Systemic treatment of children and adolescents with atopic dermatitis aged ≥2 years: a Delphi consensus project mapping expert opinion in Northern Europe.J Eur Acad Dermatol Venereol. 2022 Nov;36(11):2153-2165. doi: 10.1111/jdv.18410. Epub 2022 Jul 19. J Eur Acad Dermatol Venereol. 2022. PMID: 35793471 Free PMC article.
-
Learning from disease registries during a pandemic: Moving toward an international federation of patient registries.Clin Dermatol. 2021 May-Jun;39(3):467-478. doi: 10.1016/j.clindermatol.2021.01.018. Epub 2021 Apr 6. Clin Dermatol. 2021. PMID: 34518006 Free PMC article.
-
Use of systemic corticosteroids for atopic dermatitis: International Eczema Council consensus statement.Br J Dermatol. 2018 Mar;178(3):768-775. doi: 10.1111/bjd.15928. Epub 2018 Jan 28. Br J Dermatol. 2018. PMID: 28865094 Free PMC article.
References
-
- European Medicines Agency. Questions and answers on Sandimmun, Sandimmun Neoral and associated names (ciclosporin, 10, 25, 50 and 100 mg capsules, 100 mg/ml oral solution and 50 mg/ml concentrate for solution for infusion). 2013. http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/.... Accessed 1 Jan 2017.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous