Metabotropic and ionotropic glutamate receptors as potential targets for the treatment of alcohol use disorder
- PMID: 28242339
- PMCID: PMC5446930
- DOI: 10.1016/j.neubiorev.2017.02.024
Metabotropic and ionotropic glutamate receptors as potential targets for the treatment of alcohol use disorder
Abstract
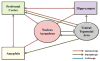
Emerging evidence indicates that dysfunctional glutamate neurotransmission is critical in the initiation and development of alcohol and drug dependence. Alcohol consumption induced downregulation of glutamate transporter 1 (GLT-1) as reported in previous studies from our laboratory. Glutamate is the major excitatory neurotransmitter in the brain, which acts via interactions with several glutamate receptors. Alcohol consumption interferes with the glutamatergic signal transmission by altering the functions of these receptors. Among the glutamate receptors involved in alcohol-drinking behavior are the metabotropic receptors such as mGluR1/5, mGluR2/3, and mGluR7, as well as the ionotropic receptors, NMDA and AMPA. Preclinical studies using agonists and antagonists implicate these glutamatergic receptors in the development of alcohol use disorder (AUD). Therefore, the purpose of this review is to discuss the neurocircuitry involving glutamate transmission in animals exposed to alcohol and further outline the role of metabotropic and ionotropic receptors in the regulation of alcohol-drinking behavior. This review provides ample information about the potential therapeutic role of glutamatergic receptors for the treatment of AUD.
Keywords: AMPA; Alcohol; Glutamate; NMDA; mGluR1/5; mGluR2/3; mGluR7.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


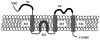
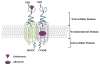
References
-
- Aal-Aaboda M, Alhaddad H, Osowik F, Nauli SM, Sari Y. Effects of (R)-(–)-5-methyl-1-nicotinoyl-2-pyrazoline on glutamate transporter 1 and cysteine/glutamate exchanger as well as ethanol drinking behavior in male, alcohol-preferring rats. Journal of neuroscience research. 2015;93:930–937. - PMC - PubMed
-
- Abrahao KP, Ariwodola OJ, Butler TR, Rau AR, Skelly MJ, Carter E, Alexander NP, McCool BA, Souza-Formigoni ML, Weiner JL. Locomotor sensitization to ethanol impairs NMDA receptor-dependent synaptic plasticity in the nucleus accumbens and increases ethanol self-administration. J Neurosci. 2013;33:4834–42. - PMC - PubMed
-
- Adams CL, Cowen MS, Short JL, Lawrence AJ. Combined antagonism of glutamate mGlu5 and adenosine A2A receptors interact to regulate alcohol-seeking in rats. Int J Neuropsychopharmacol. 2008;11:229–41. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

