Depletion of coagulation factor XII ameliorates brain pathology and cognitive impairment in Alzheimer disease mice
- PMID: 28242605
- PMCID: PMC5418637
- DOI: 10.1182/blood-2016-11-753202
Depletion of coagulation factor XII ameliorates brain pathology and cognitive impairment in Alzheimer disease mice
Abstract
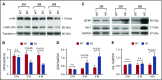
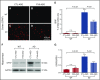
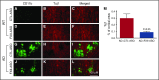
Vascular abnormalities and inflammation are found in many Alzheimer disease (AD) patients, but whether these changes play a causative role in AD is not clear. The factor XII (FXII) -initiated contact system can trigger both vascular pathology and inflammation and is activated in AD patients and AD mice. We have investigated the role of the contact system in AD pathogenesis. Cleavage of high-molecular-weight kininogen (HK), a marker for activation of the inflammatory arm of the contact system, is increased in a mouse model of AD, and this cleavage is temporally correlated with the onset of brain inflammation. Depletion of FXII in AD mice inhibited HK cleavage in plasma and reduced neuroinflammation, fibrinogen deposition, and neurodegeneration in the brain. Moreover, FXII-depleted AD mice showed better cognitive function than untreated AD mice. These results indicate that FXII-mediated contact system activation contributes to AD pathogenesis, and therefore this system may offer novel targets for AD treatment.
© 2017 by The American Society of Hematology.
Figures




Comment in
-
Alzheimer disease makes new blood contacts.Blood. 2017 May 4;129(18):2462-2463. doi: 10.1182/blood-2017-03-772087. Blood. 2017. PMID: 28473412 Free PMC article.
References
-
- Chi NF, Chien LN, Ku HL, Hu CJ, Chiou HY. Alzheimer disease and risk of stroke: a population-based cohort study. Neurology. 2013;80(8):705-711. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

