Stomach curvature is generated by left-right asymmetric gut morphogenesis
- PMID: 28242610
- PMCID: PMC5399665
- DOI: 10.1242/dev.143701
Stomach curvature is generated by left-right asymmetric gut morphogenesis
Abstract
Left-right (LR) asymmetry is a fundamental feature of internal anatomy, yet the emergence of morphological asymmetry remains one of the least understood phases of organogenesis. Asymmetric rotation of the intestine is directed by forces outside the gut, but the morphogenetic events that generate anatomical asymmetry in other regions of the digestive tract remain unknown. Here, we show in mouse and Xenopus that the mechanisms that drive the curvature of the stomach are intrinsic to the gut tube itself. The left wall of the primitive stomach expands more than the right wall, as the left epithelium becomes more polarized and undergoes radial rearrangement. These asymmetries exist across several species, and are dependent on LR patterning genes, including Foxj1, Nodal and Pitx2 Our findings have implications for how LR patterning manifests distinct types of morphological asymmetries in different contexts.
Keywords: Asymmetry; Gut; Left-right; Morphogenesis; Mouse; Pitx2; Stomach; Xenopus.
© 2017. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures
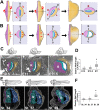

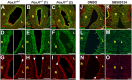

References
-
- Bamforth S. D., Bragança J., Farthing C. R., Schneider J. E., Broadbent C., Michell A. C., Clarke K., Neubauer S., Norris D., Brown N. A. et al. (2004). Cited2 controls left-right patterning and heart development through a Nodal-Pitx2c pathway. Nat. Genet. 36, 1189-1196. 10.1038/ng1446 - DOI - PubMed
-
- Campione M., Steinbeisser H., Schweickert A., Deissler K., van Bebber F., Lowe L. A., Nowotschin S., Viebahn C., Haffter P., Kuehn M. R. et al. (1999). The homeobox gene Pitx2: mediator of asymmetric left-right signaling in vertebrate heart and gut looping. Development 126, 1225-1234. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

