Quantification of labile heme in live malaria parasites using a genetically encoded biosensor
- PMID: 28242687
- PMCID: PMC5358388
- DOI: 10.1073/pnas.1615195114
Quantification of labile heme in live malaria parasites using a genetically encoded biosensor
Abstract
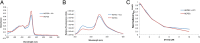
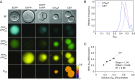
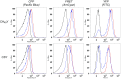
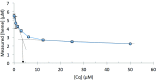
Heme is ubiquitous, yet relatively little is known about the maintenance of labile pools of this cofactor, which likely ensures its timely bioavailability for proper cellular function. Quantitative analysis of labile heme is of fundamental importance to understanding how nature preserves access to the diverse chemistry heme enables, while minimizing cellular damage caused by its redox activity. Here, we have developed and characterized a protein-based sensor that undergoes fluorescence quenching upon heme binding. By genetically encoding this sensor in the human malarial parasite, Plasmodium falciparum, we have quantified cytosolic labile heme levels in intact, blood-stage parasites. Our findings indicate that a labile heme pool (∼1.6 µM) is stably maintained throughout parasite development within red blood cells, even during a period coincident with extensive hemoglobin degradation by the parasite. We also find that the heme-binding antimalarial drug chloroquine specifically increases labile cytosolic heme, indicative of dysregulation of this homeostatic pool that may be a relevant component of the antimalarial activity of this compound class. We propose that use of this technology under various environmental perturbations in P. falciparum can yield quantitative insights into fundamental heme biology.
Keywords: Plasmodium falciparum; genetically encoded biosensor; heme; heme sensor; malaria.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


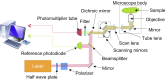

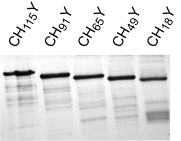
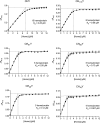

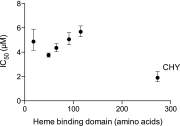


References
-
- Ziegler J, Linck R, Wright DW. Heme aggregation inhibitors: Antimalarial drugs targeting an essential biomineralization process. Curr Med Chem. 2001;8(2):171–189. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

