Manganese scavenging and oxidative stress response mediated by type VI secretion system in Burkholderia thailandensis
- PMID: 28242693
- PMCID: PMC5358365
- DOI: 10.1073/pnas.1614902114
Manganese scavenging and oxidative stress response mediated by type VI secretion system in Burkholderia thailandensis
Abstract
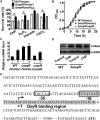
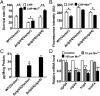
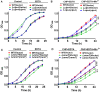
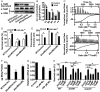
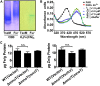
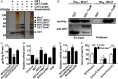
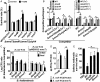
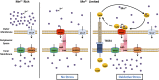
Type VI secretion system (T6SS) is a versatile protein export machinery widely distributed in Gram-negative bacteria. Known to translocate protein substrates to eukaryotic and prokaryotic target cells to cause cellular damage, the T6SS has been primarily recognized as a contact-dependent bacterial weapon for microbe-host and microbial interspecies competition. Here we report contact-independent functions of the T6SS for metal acquisition, bacteria competition, and resistance to oxidative stress. We demonstrate that the T6SS-4 in Burkholderia thailandensis is critical for survival under oxidative stress and is regulated by OxyR, a conserved oxidative stress regulator. The T6SS-4 is important for intracellular accumulation of manganese (Mn2+) under oxidative stress. Next, we identified a T6SS-4-dependent Mn2+-binding effector TseM, and its interacting partner MnoT, a Mn2+-specific TonB-dependent outer membrane transporter. Similar to the T6SS-4 genes, expression of mnoT is regulated by OxyR and is induced under oxidative stress and low Mn2+ conditions. Both TseM and MnoT are required for efficient uptake of Mn2+ across the outer membrane under Mn2+-limited and -oxidative stress conditions. The TseM-MnoT-mediated active Mn2+ transport system is also involved in contact-independent bacteria-bacteria competition and bacterial virulence. This finding provides a perspective for understanding the mechanisms of metal ion uptake and the roles of T6SS in bacteria-bacteria competition.
Keywords: Burkholderia; ion uptake; outer membrane transporter; oxidative stress; type VI secretion.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



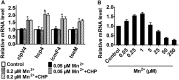


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

