A novel role for the Wnt inhibitor APCDD1 in adipocyte differentiation: Implications for diet-induced obesity
- PMID: 28242765
- PMCID: PMC5391760
- DOI: 10.1074/jbc.M116.758078
A novel role for the Wnt inhibitor APCDD1 in adipocyte differentiation: Implications for diet-induced obesity
Abstract
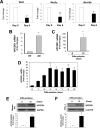
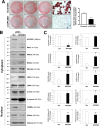
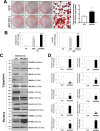
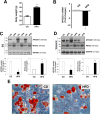
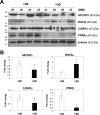
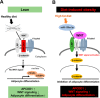
Impaired adipogenic differentiation during diet-induced obesity (DIO) promotes adipocyte hypertrophy and inflammation, thereby contributing to metabolic disease. Adenomatosis polyposis coli down-regulated 1 (APCDD1) has recently been identified as an inhibitor of Wnt signaling, a key regulator of adipogenic differentiation. Here we report a novel role for APCDD1 in adipogenic differentiation via repression of Wnt signaling and an epigenetic linkage between miR-130 and APCDD1 in DIO. APCDD1 expression was significantly up-regulated in mature adipocytes compared with undifferentiated preadipocytes in both human and mouse subcutaneous adipose tissues. siRNA-based silencing of APCDD1 in 3T3-L1 preadipocytes markedly increased the expression of Wnt signaling proteins (Wnt3a, Wnt5a, Wnt10b, LRP5, and β-catenin) and inhibited the expression of adipocyte differentiation markers (CCAAT/enhancer-binding protein α (C/EBPα) and peroxisome proliferator-activated receptor γ (PPARγ)) and lipid droplet accumulation, whereas adenovirus-mediated overexpression of APCDD1 enhanced adipogenic differentiation. Notably, DIO mice exhibited reduced APCDD1 expression and increased Wnt expression in both subcutaneous and visceral adipose tissues and impaired adipogenic differentiation in vitro Mechanistically, we found that miR-130, whose expression is up-regulated in adipose tissues of DIO mice, could directly target the 3'-untranslated region of the APCDD1 gene. Furthermore, transfection of an miR-130 inhibitor in preadipocytes enhanced, whereas an miR-130 mimic blunted, adipogenic differentiation, suggesting that miR-130 contributes to impaired adipogenic differentiation during DIO by repressing APCDD1 expression. Finally, human subcutaneous adipose tissues isolated from obese individuals exhibited reduced expression of APCDD1, C/EBPα, and PPARγ compared with those from non-obese subjects. Taken together, these novel findings suggest that APCDD1 positively regulates adipogenic differentiation and that its down-regulation by miR-130 during DIO may contribute to impaired adipogenic differentiation and obesity-related metabolic disease.
Keywords: APCDD1; Wnt signaling; adipocyte; adipose tissue; differentiation; miR-130; obesity.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures

References
-
- MacDougald O. A., and Mandrup S. (2002) Adipogenesis: forces that tip the scales. Trends Endocrinol. Metab. 13, 5–11 - PubMed
-
- Klöting N., and Blüher M. (2014) Adipocyte dysfunction, inflammation and metabolic syndrome. Rev. Endocr. Metab. Disord. 15, 277–287 - PubMed
-
- Lönn M., Mehlig K., Bengtsson C., and Lissner L. (2010) Adipocyte size predicts incidence of type 2 diabetes in women. FASEB J. 24, 326–331 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

