Activated T Lymphocytes are Essential Drivers of Pathological Remodeling in Ischemic Heart Failure
- PMID: 28242779
- PMCID: PMC5331621
- DOI: 10.1161/CIRCHEARTFAILURE.116.003688
Activated T Lymphocytes are Essential Drivers of Pathological Remodeling in Ischemic Heart Failure
Abstract
Background: Inappropriately sustained inflammation is a hallmark of chronic ischemic heart failure (HF); however, the pathophysiological role of T lymphocytes is unclear.
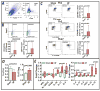
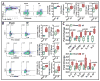
Methods and results: Permanent coronary ligation was performed in adult C57BL/6 mice. When compared with sham-operated mice, mice with HF (8 weeks after ligation) exhibited the following features: (1) significant (P<0.05) expansion of circulating CD3+CD8+ cytotoxic and CD3+CD4+ helper (Th) T lymphocytes, together with increased Th1, Th2, Th17, and regulatory T-cell (Treg) CD4+ subsets; (2) significant expansion of CD8+ and CD4+ T cells in failing myocardium, with increased Th1, Th2, Th17, and Treg CD4+ subsets, marked reduction of the Th1/Th2 ratio, augmentation of the Th17/Treg ratio, and upregulation of Th2 cytokines; and (3) significantly increased Th1, Th2, Th17 cells, and Tregs, in the spleen and mediastinal lymph nodes, with expansion of splenic antigen-experienced effector and memory CD4+ T cells. Antibody-mediated CD4+ T-cell depletion in HF mice (starting 4 weeks after ligation) reduced cardiac infiltration of CD4+ T cells and prevented progressive left ventricular dilatation and hypertrophy, whereas adoptive transfer of splenic CD4+ T cells (and, to a lesser extent, cardiac CD3+ T cells) from donor mice with HF induced long-term left ventricular dysfunction, fibrosis, and hypertrophy in naive recipient mice.
Conclusions: CD4+ T lymphocytes are globally expanded and activated in chronic ischemic HF, with Th2 (versus Th1) and Th17 (versus Treg) predominance in failing hearts, and with expansion of memory T cells in the spleen. Cardiac and splenic T cells in HF are primed to induce cardiac injury and remodeling, and retain this memory on adoptive transfer.
Keywords: T lymphocytes; adaptive immunity; adoptive transfer; heart failure; inflammation.
© 2017 American Heart Association, Inc.
Figures






References
-
- Mann DL. Inflammatory mediators and the failing heart: Past, present, and the foreseeable future. Circ Res. 2002;91:988–998. - PubMed
-
- Prabhu SD, Chandrasekar B, Murray DR, Freeman GL. Beta-adrenergic blockade in developing heart failure: Effects on myocardial inflammatory cytokines, nitric oxide, and remodeling. Circulation. 2000;101:2103–2109. - PubMed
-
- Chung ES, Packer M, Lo KH, Fasanmade AA, Willerson JT. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-α, in patients with moderate-to-severe heart failure: Results of the anti-TNF therapy against congestive heart failure (ATTACH) trial. Circulation. 2003;107:3133–3140. - PubMed
-
- Mann DL, McMurray JJ, Packer M, Swedberg K, Borer JS, Colucci WS, Djian J, Drexler H, Feldman A, Kober L, Krum H, Liu P, Nieminen M, Tavazzi L, van Veldhuisen DJ, Waldenstrom A, Warren M, Westheim A, Zannad F, Fleming T. Targeted anticytokine therapy in patients with chronic heart failure: Results of the randomized etanercept worldwide evaluation (renewal) Circulation. 2004;109:1594–1602. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

