Deubiquitinase USP18 Loss Mislocalizes and Destabilizes KRAS in Lung Cancer
- PMID: 28242811
- PMCID: PMC5635999
- DOI: 10.1158/1541-7786.MCR-16-0369
Deubiquitinase USP18 Loss Mislocalizes and Destabilizes KRAS in Lung Cancer
Abstract
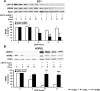
KRAS is frequently mutated in lung cancers and is associated with aggressive biology and chemotherapy resistance. Therefore, innovative approaches are needed to treat these lung cancers. Prior work implicated the IFN-stimulated gene 15 (ISG15) deubiquitinase (DUB) USP18 as having antineoplastic activity by regulating lung cancer growth and oncoprotein stability. This study demonstrates that USP18 affects the stability of the KRAS oncoprotein. Interestingly, loss of USP18 reduced KRAS expression, and engineered gain of USP18 expression increased KRAS protein levels in lung cancer cells. Using the protein synthesis inhibitor cycloheximide, USP18 knockdown significantly reduced the half-life of KRAS, but gain of USP18 expression significantly increased its stability. Intriguingly, loss of USP18 altered KRAS subcellular localization by mislocalizing KRAS from the plasma membrane. To explore the biologic consequences, immunohistochemical (IHC) expression profiles of USP18 were compared in lung cancers of KrasLA2/+ versus cyclin E engineered mouse models. USP18 expression was higher in Kras-driven murine lung cancers, indicating a link between KRAS and USP18 expression in vivo To solidify this association, loss of Usp18 in KrasLA2/+ /Usp18-/- mice was found to significantly reduce lung cancers as compared with parental KrasLA2/+ mice. Finally, translational relevance was confirmed in a human lung cancer panel by showing that USP18 IHC expression was significantly higher in KRAS-mutant versus wild-type lung adenocarcinomas.Implications: Taken together, this study highlights a new way to combat the oncogenic consequences of activated KRAS in lung cancer by inhibiting the DUB USP18. Mol Cancer Res; 15(7); 905-14. ©2017 AACR.
©2017 American Association for Cancer Research.
Conflict of interest statement
No potential conflicts of interests exist.
Figures





References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. - PubMed
-
- Macerecilli M, Caramella C, Faivre L, Besse B, Pianchard D, Polo V, et al. Does KRAS mutational status predict chemoresistance in advanced non-small cell lung cancer (NSCLC)? J Lung Cancer. 2014;83:383–388. - PubMed
-
- Frezza M, Schmitt S, Dou QP. Targeting the ubiquitin-proteasome pathway: an emerging concept in cancer therapy. Curr Top in Med Chem. 2011;11:2888–2905. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

