Gender differences in hypoxic acclimatization in cyclooxygenase-2-deficient mice
- PMID: 28242826
- PMCID: PMC5328777
- DOI: 10.14814/phy2.13148
Gender differences in hypoxic acclimatization in cyclooxygenase-2-deficient mice
Abstract
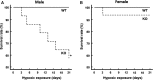
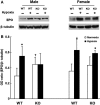
The aim of this study was to determine the effect of cyclooxygenase-2 (COX-2) gene deletion on the adaptive responses during prolonged moderate hypobaric hypoxia. Wild-type (WT) and COX-2 knockout (KO) mice of both genders (3 months old) were exposed to hypobaric hypoxia (~0.4 ATM) or normoxia for 21 days and brain capillary densities were determined. Hematocrit was measured at different time intervals; brain hypoxia-inducible factor -1α (HIF-1α), angiopoietin 2 (Ang-2), brain erythropoietin (EPO), and kidney EPO were measured under normoxic and hypoxic conditions. There were no gender differences in hypoxic acclimatization in the WT mice and similar adaptive responses were observed in the female KO mice. However, the male KO mice exhibited progressive vulnerability to prolonged hypoxia. Compared to the WT and female KO mice, the male COX-2 KO mice had significantly lower survival rate and decreased erythropoietic and polycythemic responses, diminished cerebral angiogenesis, decreased brain accumulation of HIF-1α, and attenuated upregulation of VEGF, EPO, and Ang-2 during hypoxia. Our data suggest that there are physiologically important gender differences in hypoxic acclimatization in COX-2-deficient mice. The COX-2 signaling pathway appears to be required for acclimatization in oxygen-limiting environments only in males, whereas female COX-2-deficient mice may be able to access COX-2-independent mechanisms to achieve hypoxic acclimatization.
Keywords: Capillary density; hypoxia‐induced angiogenesis; hypoxic adaptation; prolonged hypoxia; sex differences.
© 2017 Case Western Reserve University. Physiological Reports published by Wiley Periodicals, Inc. on behalf of The Physiological Society and the American Physiological Society.
Figures




Similar articles
-
HIF-1α/COX-2 expression and mouse brain capillary remodeling during prolonged moderate hypoxia and subsequent re-oxygenation.Brain Res. 2014 Jun 20;1569:41-7. doi: 10.1016/j.brainres.2014.04.035. Epub 2014 May 2. Brain Res. 2014. PMID: 24796880 Free PMC article.
-
Increased HIF-1α and HIF-2α accumulation, but decreased microvascular density, in chronic hyperoxia and hypercapnia in the mouse cerebral cortex.Adv Exp Med Biol. 2013;789:29-35. doi: 10.1007/978-1-4614-7411-1_5. Adv Exp Med Biol. 2013. PMID: 23852473
-
Cerebral adaptations to chronic anemia in a model of erythropoietin-deficient mice exposed to hypoxia.Am J Physiol Regul Integr Comp Physiol. 2009 Mar;296(3):R801-11. doi: 10.1152/ajpregu.00119.2008. Epub 2008 Dec 24. Am J Physiol Regul Integr Comp Physiol. 2009. PMID: 19109375
-
Physiologic angiodynamics in the brain.Antioxid Redox Signal. 2007 Sep;9(9):1363-71. doi: 10.1089/ars.2007.1713. Antioxid Redox Signal. 2007. PMID: 17627476 Review.
-
Erythropoietin and the hypoxic brain.J Exp Biol. 2004 Aug;207(Pt 18):3233-42. doi: 10.1242/jeb.01049. J Exp Biol. 2004. PMID: 15299044 Review.
References
-
- Benderro, G. F. , and LaManna J. C.. 2013. Kidney EPO expression during chronic hypoxia in aged mice. Adv. Exp. Med. Biol. 765:9–14. - PubMed
-
- Bergeron, M. , Gidday J. M., Yu A. Y., Semenza G. L., Ferriero D. M., and Sharp F. R.. 2000. Role of hypoxia‐inducible factor‐1 in hypoxia‐induced ischemic tolerance in neonatal rat brain. Ann . Neurol. 48:285–296. - PubMed
-
- Casibang, M. , Purdom S., Jakowlew S., Neckers L., Zia F., Ben‐Av P., et al. 2001. Prostaglandin E2 and vasoactive intestinal peptide increase vascular endothelial cell growth factor mRNAs in lung cancer cells. Lung Cancer 31:203–212. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

