Effects of functional decoupling of a leg in a model of stick insect walking incorporating three ipsilateral legs
- PMID: 28242829
- PMCID: PMC5328780
- DOI: 10.14814/phy2.13154
Effects of functional decoupling of a leg in a model of stick insect walking incorporating three ipsilateral legs
Abstract
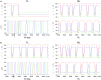
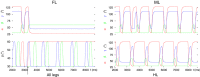
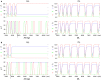
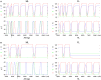
Legged locomotion is a fundamental form of activity of insects during which the legs perform coordinated movements. Sensory signals conveying position, velocity and load of a leg are sent between the thoracic ganglia where the local control networks of the leg muscles are situated. They affect the actual state of the local control networks, hence the stepping of the legs. Sensory coordination in stepping has been intensively studied but important details of its neuronal mechanisms are still unclear. One possibility to tackle this problem is to study what happens to the coordination if a leg is, reversibly or irreversibly, deprived of its normal function. There are numerous behavioral studies on this topic but they could not fully uncover the underlying neuronal mechanisms. Another promising approach to make further progress here can be the use of appropriate models that help elucidate those coordinating mechanisms. We constructed a model of three ipsilateral legs of a stick insect that can mimic coordinated stepping of these legs. We used this model to investigate the possible effects of decoupling a leg. We found that decoupling of the front or the hind leg did not disrupt the coordinated walking of the two remaining legs. However, decoupling of the middle leg yielded mixed results. Both disruption and continuation of coordinated stepping of the front and hind leg occurred. These results agree with the majority of corresponding experimental findings. The model suggests a number of possible mechanisms of decoupling that might bring about the changes.
Keywords: Insect locomotion; network model; neuromuscular control.
© 2017 The Authors. Physiological Reports published by Wiley Periodicals, Inc. on behalf of The Physiological Society and the American Physiological Society.
Figures






References
-
- Bässler, U. 1977. Sensory control of leg movement in the stick insect Carausius morosus. Biol. Cybern. 25:61–72. - PubMed
-
- Bässler, U . 1983. Neural basis of elementary behavior in stick insects. Springer Verlag, Berlin‐ Heidelberg, Germany.
-
- Bässler, U . 1993. The walking‐ (and searching‐) pattern generator of stick insects, a modular system composed of reflex chains and endogenous oscillators. Biol. Cybern. 69:305–317.
-
- Berg, E. , Büschges A., and Schmidt J.. 2011. Single perturbations cause sustained changes in searching behavior in stick insects. J. Exp. Biol. 216:1064–1074. - PubMed
-
- Borgmann, A. , Scharstein H., and Buschges A.. 2007. Intersegmental coordination: influence of a single walking leg on the neighboring segments in the stick insect walking system. J. Neurophysiol. 98:1685–1696. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

