Neurons derived from patients with bipolar disorder divide into intrinsically different sub-populations of neurons, predicting the patients' responsiveness to lithium
- PMID: 28242870
- PMCID: PMC5573640
- DOI: 10.1038/mp.2016.260
Neurons derived from patients with bipolar disorder divide into intrinsically different sub-populations of neurons, predicting the patients' responsiveness to lithium
Abstract
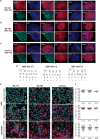
Bipolar disorder (BD) is a progressive psychiatric disorder with more than 3% prevalence worldwide. Affected individuals experience recurrent episodes of depression and mania, disrupting normal life and increasing the risk of suicide greatly. The complexity and genetic heterogeneity of psychiatric disorders have challenged the development of animal and cellular models. We recently reported that hippocampal dentate gyrus (DG) neurons differentiated from induced pluripotent stem cell (iPSC)-derived fibroblasts of BD patients are electrophysiologically hyperexcitable. Here we used iPSCs derived from Epstein-Barr virus-immortalized B-lymphocytes to verify that the hyperexcitability of DG-like neurons is reproduced in this different cohort of patients and cells. Lymphocytes are readily available for research with a large number of banked lines with associated patient clinical description. We used whole-cell patch-clamp recordings of over 460 neurons to characterize neurons derived from control individuals and BD patients. Extensive functional analysis showed that intrinsic cell parameters are very different between the two groups of BD neurons, those derived from lithium (Li)-responsive (LR) patients and those derived from Li-non-responsive (NR) patients, which led us to partition our BD neurons into two sub-populations of cells and suggested two different subdisorders. Training a Naïve Bayes classifier with the electrophysiological features of patients whose responses to Li are known allows for accurate classification with more than 92% success rate for a new patient whose response to Li is unknown. Despite their very different functional profiles, both populations of neurons share a large, fast after-hyperpolarization (AHP). We therefore suggest that the large, fast AHP is a key feature of BD and a main contributor to the fast, sustained spiking abilities of BD neurons. Confirming our previous report with fibroblast-derived DG neurons, chronic Li treatment reduced the hyperexcitability in the lymphoblast-derived LR group but not in the NR group, strengthening the validity and utility of this new human cellular model of BD.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




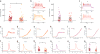
References
-
- Angst J. The emerging epidemiology of hypomania and bipolar II disorder. J Affect Disord. 1998;50:143–151. - PubMed
-
- Hirschfeld RM, Calabrese JR, Weissman MM, Reed M, Davies MA, Frye MA, et al. Screening for bipolar disorder in the community. J Clin Psychiatry. 2003;64:53–59. - PubMed
-
- Belmaker RH. Bipolar disorder. N Engl J Med. 2004;351:476–486. - PubMed
-
- Judd LL, Akiskal HS, Schettler PJ, Endicott J, Maser J, Solomon DA, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59:530–537. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

