Oral and transdermal drug delivery systems: role of lipid-based lyotropic liquid crystals
- PMID: 28243062
- PMCID: PMC5315216
- DOI: 10.2147/DDDT.S103505
Oral and transdermal drug delivery systems: role of lipid-based lyotropic liquid crystals
Abstract
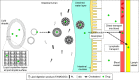
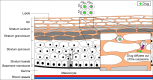
Liquid crystal (LC) dosage forms, particularly those using lipid-based lyotropic LCs (LLCs), have generated considerable interest as potential drug delivery systems. LCs have the physical properties of liquids but retain some of the structural characteristics of crystalline solids. They are compatible with hydrophobic and hydrophilic compounds of many different classes and can protect even biologicals and nucleic acids from degradation. This review, focused on research conducted over the past 5 years, discusses the structural evaluation of LCs and their effects in drug formulations. The structural classification of LLCs into lamellar, hexagonal and micellar cubic phases is described. The structures of these phases are influenced by the addition of surfactants, which include a variety of nontoxic, biodegradable lipids; these also enhance drug solubility. LLC structure influences drug localization, particle size and viscosity, which, in turn, determine drug delivery properties. Through several specific examples, we describe the applications of LLCs in oral and topical drug formulations, the latter including transdermal and ocular delivery. In oral LLC formulations, micelle compositions and the resulting LLC structures can determine drug solubilization and stability as well as intestinal transport and absorption. Similarly, in topical LLC formulations, composition can influence whether the drug is retained in the skin or delivered transdermally. Owing to their enhancement of drug stability and promotion of controlled drug delivery, LLCs are becoming increasingly popular in pharmaceutical formulations.
Keywords: controlled release; drug delivery; drug localization; liquid crystal; lyotropic; surfactants.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures


Similar articles
-
Oral Drug Delivery via Intestinal Lymphatic Transport Utilizing Lipid-Based Lyotropic Liquid Crystals.Liquids (Basel). 2023 Dec;3(4):456-468. doi: 10.3390/liquids3040029. Epub 2023 Nov 20. Liquids (Basel). 2023. PMID: 38711572 Free PMC article.
-
Interactions of biomacromolecules with reverse hexagonal liquid crystals: drug delivery and crystallization applications.J Colloid Interface Sci. 2011 Apr 15;356(2):375-86. doi: 10.1016/j.jcis.2011.01.047. Epub 2011 Jan 21. J Colloid Interface Sci. 2011. PMID: 21315366
-
Advances in lyotropic liquid crystal systems for skin drug delivery.Expert Opin Drug Deliv. 2020 Dec;17(12):1781-1805. doi: 10.1080/17425247.2020.1819979. Epub 2020 Sep 18. Expert Opin Drug Deliv. 2020. PMID: 32886531 Review.
-
Lyotropic liquid crystalline phases: Drug delivery and biomedical applications.Int J Pharm. 2023 Nov 25;647:123546. doi: 10.1016/j.ijpharm.2023.123546. Epub 2023 Oct 25. Int J Pharm. 2023. PMID: 37884213 Review.
-
Lyotropic liquid crystal-based transcutaneous peptide delivery system: Evaluation of skin permeability and potential for transcutaneous vaccination.Acta Biomater. 2022 Jan 15;138:273-284. doi: 10.1016/j.actbio.2021.11.008. Epub 2021 Nov 10. Acta Biomater. 2022. PMID: 34774785
Cited by
-
Chitosan/Lactic Acid Systems: Liquid Crystalline Behavior, Rheological Properties, and Riboflavin Release In Vitro.Int J Mol Sci. 2022 Oct 30;23(21):13207. doi: 10.3390/ijms232113207. Int J Mol Sci. 2022. PMID: 36362002 Free PMC article.
-
Sustain-release lipid-liquid crystal formulations of pexiganan against Helicobacter pylori infection: in vitro evaluation in C57BL/6 mice.BMC Pharmacol Toxicol. 2024 Jan 11;25(1):9. doi: 10.1186/s40360-024-00731-z. BMC Pharmacol Toxicol. 2024. PMID: 38212864 Free PMC article.
-
Formation of Self-Assembled Liquid Crystalline Nanoparticles and Absorption Enhancement of Ω-3s by Phospholipids and Oleic Acids.Pharmaceutics. 2021 Dec 28;14(1):68. doi: 10.3390/pharmaceutics14010068. Pharmaceutics. 2021. PMID: 35056964 Free PMC article.
-
Molecular Structuring and Phase Transition of Lipid-Based Formulations upon Water Dispersion: A Coarse-Grained Molecular Dynamics Simulation Approach.Mol Pharm. 2017 Dec 4;14(12):4145-4153. doi: 10.1021/acs.molpharmaceut.7b00397. Epub 2017 Aug 29. Mol Pharm. 2017. PMID: 28799773 Free PMC article.
-
Liquid Crystalline Phases for Enhancement of Oral Bioavailability.AAPS PharmSciTech. 2021 Feb 22;22(3):81. doi: 10.1208/s12249-021-01951-w. AAPS PharmSciTech. 2021. PMID: 33619612 Review.
References
-
- Hitesh J, Rushikesh G, Gauri J, Jagruti M, Nirali T. Liquid crystal as accelerant in drug absorption from topical formulations. Int Res J Pharm. 2011;2(4):86–89.
-
- Sekhon BS. Surfactants: pharmaceutical and medicinal aspects. J Pharm Technol Res Manag. 2013;1:11–36.
-
- Guo C, Wang J, Cao F, Lee RJ, Zhai G. Lyotropic liquid crystal systems in drug delivery. Drug Discov Today. 2010;15(23–24):1032–1040. - PubMed
-
- Nagarajan R, Ruckenstein E. Critical micelle concentration: a transition point for micellar size distribution. J Colloid Interface Sci. 1977;60(2):221–231.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

