Performance of the FreeStyle Libre Flash glucose monitoring system in patients with type 1 and 2 diabetes mellitus
- PMID: 28243449
- PMCID: PMC5316912
- DOI: 10.1136/bmjdrc-2016-000320
Performance of the FreeStyle Libre Flash glucose monitoring system in patients with type 1 and 2 diabetes mellitus
Abstract
Objective: To evaluate the performance of the FreeStyle Libre Flash continuous glucose monitoring (FSL-CGM) system against established central laboratory methods.
Research design and methods: 20 subjects (8 type 1 diabetes mellitus, 12 type 2 diabetes mellitus) were analyzed. FSL-CGM sensor measurements (inserted in arm and abdomen) were compared with capillary blood glucose results analyzed with StatStrip as semigold standard. The glucose response after a standardized oral glucose load was measured by FSL-CGM and capillary samples analyzed by perchloric acid hexokinase (PCA-HK) method, StatStrip and FSL test strip (FSLC), and a commonly used CGM system (iPro2).
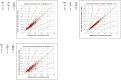
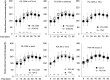
Results: FSL-CGM arm sensor readings showed 85.5% of paired readings falling within Clarke Error Grid (ISO 15197:2013) zone A when compared with StatStrip. For FSL-CGM abdomen and FSLC, these percentages were 64% and 98%, respectively. The overall correlation of FSL-CGM in the arm and the StatStrip indicates a performance with lower results with the FSL-CGM in the arm than expected based on the StatStrip in the lower glucose ranges, and higher results than expected in the higher ranges. Following a standardized glucose load, a slower rise in glucose level was observed for FSL-CGM arm as compared with PCA-HK, StatStrip, FSLC, and iPro2 during the first 45-60 min after glucose load ingestion.
Conclusions: Certain matters need attention while using the FSL-CGM in daily life including the observed lower values in the lower ranges, and the underestimation of the effect of a meal on glucose response. These effects of such deviations can partly be overcome by optimizing the available user instructions.
Trial registration number: TC5348; results.
Keywords: Blood Glucose Self-Monitoring; Continuous Glucose Monitoring; Endocrinology Diabetes; Glucose Control.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- ISO 15197. In vitro diagnostic test systems—requirements for blood-glucose monitoring systems for self-testing in managing diabetes mellitus 2013. https://http://www.iso.org/obp/ui (accessed 29 Feb 2016).
-
- Riemsma R, Corro Ramos I, Birnie R et al. . Integrated sensor-augmented pump therapy systems [the MiniMed(R) Paradigm Veo system and the Vibe and G4(R) PLATINUM CGM (continuous glucose monitoring) system] for managing blood glucose levels in type 1 diabetes: a systematic review and economic evaluation. Health Technol Assess 2016;20:1–252. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
