Imaging windows for long-term intravital imaging: General overview and technical insights
- PMID: 28243510
- PMCID: PMC5312719
- DOI: 10.4161/intv.29917
Imaging windows for long-term intravital imaging: General overview and technical insights
Abstract
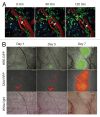
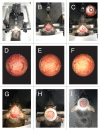
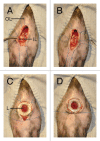
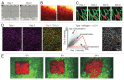
Intravital microscopy is increasingly used to visualize and quantitate dynamic biological processes at the (sub)cellular level in live animals. By visualizing tissues through imaging windows, individual cells (e.g., cancer, host, or stem cells) can be tracked and studied over a time-span of days to months. Several imaging windows have been developed to access tissues including the brain, superficial fascia, mammary glands, liver, kidney, pancreas, and small intestine among others. Here, we review the development of imaging windows and compare the most commonly used long-term imaging windows for cancer biology: the cranial imaging window, the dorsal skin fold chamber, the mammary imaging window, and the abdominal imaging window. Moreover, we provide technical details, considerations, and trouble-shooting tips on the surgical procedures and microscopy setups for each imaging window and explain different strategies to assure imaging of the same area over multiple imaging sessions. This review aims to be a useful resource for establishing the long-term intravital imaging procedure.
Keywords: abdominal imaging window; cranial imaging window; dorsal skinfold chamber; intravital microscopy; mammary imaging window; surgery.
Figures


References
-
- Condeelis J, Weissleder R. . In vivo imaging in cancer. Cold Spring Harb Perspect Biol 2010; 2:a003848; http://dx.doi.org/ 10.1101/cshperspect.a003848; PMID: 20861158 - DOI - PMC - PubMed
-
- Ritsma L, Ponsioen B, Van Rheenen J. . Intravital imaging of cell signaling in mice. IntraVital 2012; 1:2 - 10; http://dx.doi.org/ 10.4161/intv.20802 - DOI
-
- Pittet MJ, Weissleder R. . Intravital imaging. Cell 2011; 147:983 - 91; http://dx.doi.org/ 10.1016/j.cell.2011.11.004; PMID: 22118457 - DOI - PMC - PubMed
-
- Ellenbroek SI, van Rheenen J. . Imaging hallmarks of cancer in living mice. Nat Rev Cancer 2014; 14:406 - 18; http://dx.doi.org/ 10.1038/nrc3742; PMID: 24854083 - DOI - PubMed
-
- Fein MR, Egeblad M. . Caught in the act: revealing the metastatic process by live imaging. Dis Model Mech 2013; 6:580 - 93; http://dx.doi.org/ 10.1242/dmm.009282; PMID: 23616077 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
