Homologation of α-aryl amino acids through quinone-catalyzed decarboxylation/Mukaiyama-Mannich addition
- PMID: 28243665
- PMCID: PMC5604228
- DOI: 10.1039/c7cc00485k
Homologation of α-aryl amino acids through quinone-catalyzed decarboxylation/Mukaiyama-Mannich addition
Abstract
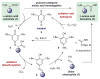
A new method for amino acid homologation by way of formal C-C bond functionalization is reported. This method utilizes a 2-step/1-pot protocol to convert α-amino acids to their corresponding N-protected β-amino esters through quinone-catalyzed oxidative decarboxylation/in situ Mukaiyama-Mannich addition. The scope and limitations of this chemistry are presented. This methodology provides an alternative to the classical Arndt-Eistert homologation for accessing β-amino acid derivatives. The resulting N-protected amine products can be easily deprotected to afford the corresponding free amines.
Figures
References
-
-
For a related review, see: Cheng RP, Gellman SH, DeGrado WF. Chem Rev. 2001;101:3219.
-
-
-
For selected reviews, see: Juaristi E, Soloshonok VA. Enantioselective Synthesis of β-Aminoacids. 2. Wiley-Interscience; New York: 2005. Bandala Y, Juaristi E. Vol. 1. Wiley-VCH Verlag GmbH & Co. KGaA; 2009. p. 291.Sleebs BE, Van Nguyen TT, Hughes AB. Org Prep Proced Int. 2009;41:429.Weiner B, Szymanski W, Janssen DB, Minnaard AJ, Feringa BL. Chem Soc Rev. 2010;39:1656.Chhiba V, Bode M, Mathiba K, Brady D. Wiley-VCH Verlag GmbH & Co. KGaA; 2014. p. 297.
-
-
-
For selected recent examples, see: Cheng J, Qi X, Li M, Chen P, Liu G. J Am Chem Soc. 2015;137:2480.Faulkner A, Scott JS, Bower JF. J Am Chem Soc. 2015;137:7224.
-
-
- Arndt F, Eistert B. Ber dtsch Chem Ges. 1935;68:200.
- Ellmerer-Mueller EP, Broessner D, Maslouh N, Tako A. Helv Chim Acta. 1998;81:59.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources