Tenapanor Treatment of Patients With Constipation-Predominant Irritable Bowel Syndrome: A Phase 2, Randomized, Placebo-Controlled Efficacy and Safety Trial
- PMID: 28244495
- PMCID: PMC5418559
- DOI: 10.1038/ajg.2017.41
Tenapanor Treatment of Patients With Constipation-Predominant Irritable Bowel Syndrome: A Phase 2, Randomized, Placebo-Controlled Efficacy and Safety Trial
Abstract
Objectives: Tenapanor is a first-in-class, small-molecule inhibitor of the gastrointestinal sodium/hydrogen exchanger NHE3. This study assessed the efficacy and safety of tenapanor in patients with constipation-predominant irritable bowel syndrome (IBS-C).
Methods: In this phase 2, double-blind study, patients with IBS-C (Rome III criteria) were randomized (1:1:1:1) to receive tenapanor 5 mg, 20 mg, or 50 mg b.i.d., or placebo b.i.d. for 12 weeks. The primary end point was the complete spontaneous bowel movement (CSBM) responder rate, defined as the proportion of patients reporting an increase from baseline of ≥1 CSBM/week for ≥6/12 treatment weeks. Secondary end points included abdominal symptom responder rates (≥30% score improvement from baseline for ≥6/12 weeks) and a composite responder rate (CSBM and abdominal pain response in the same week for ≥6/12 weeks).
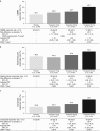
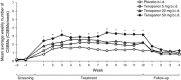
Results: Overall, 356 patients were randomized (mean age: 45.7 years; 86.8% women) and 304 completed the study. The CSBM responder rate was significantly higher in the tenapanor 50 mg b.i.d. group than in the placebo group (60.7 vs. 33.7%; P<0.001), as was the composite responder rate (50.0 vs. 23.6%; P<0.001). Responder rates for abdominal symptoms (pain, discomfort, bloating, cramping, and fullness) were significantly higher in the tenapanor 50 mg b.i.d. group than in the placebo group (all P<0.05). Diarrhea was the most frequent adverse event (tenapanor b.i.d.: 20 mg, 12.4%; 50 mg, 11.2%).
Conclusions: Tenapanor 50 mg b.i.d. significantly increased stool frequency and reduced abdominal symptoms in patients with IBS-C. Further research into tenapanor as a potential treatment for these patients is justified.
Conflict of interest statement
Figures

Comment in
-
[Diversion].Z Gastroenterol. 2017 Nov;55(11):1239-1240. doi: 10.1055/s-0043-119658. Epub 2017 Nov 15. Z Gastroenterol. 2017. PMID: 29141271 German. No abstract available.
References
-
- Longstreth GF, Thompson WG, Chey WD et al. Functional bowel disorders. Gastroenterology 2006;130:1480–1491. - PubMed
-
- Chey WD, Kurlander J, Eswaran S. Irritable bowel syndrome: a clinical review. JAMA 2015;313:949–958. - PubMed
-
- Drossman DA, Camilleri M, Mayer EA et al. AGA technical review on irritable bowel syndrome. Gastroenterology 2002;123:2108–2131. - PubMed
-
- Pare P, Gray J, Lam S et al. Health-related quality of life, work productivity, and health care resource utilization of subjects with irritable bowel syndrome: baseline results from LOGIC (Longitudinal Outcomes Study of Gastrointestinal Symptoms in Canada), a naturalistic study. Clin Ther 2006;28:1726–1735. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

