Optimizing the analysis strategy for the CANVAS Program: A prespecified plan for the integrated analyses of the CANVAS and CANVAS-R trials
- PMID: 28244644
- PMCID: PMC5485085
- DOI: 10.1111/dom.12924
Optimizing the analysis strategy for the CANVAS Program: A prespecified plan for the integrated analyses of the CANVAS and CANVAS-R trials
Abstract
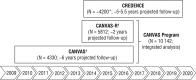
Two large cardiovascular outcome trials of canagliflozin, comprising the CANVAS Program, will complete in early 2017: the CANagliflozin cardioVascular Assessment Study (CANVAS) and the CANagliflozin cardioVascular Assessment Study-Renal (CANVAS-R). Accruing data for the sodium glucose co-transporter 2 (SGLT2) inhibitor class has identified questions and opportunities that were not apparent when the trials were designed. Accordingly, a series of modifications have been made to the planned analyses. These updates will ensure that the data from the CANVAS Program will maximize advances in scientific knowledge and patient care. The specification of the analysis strategy prior to knowledge of the trial results, their design by the independent scientific trial Steering Committee, the detailed a priori definition of the analysis plans, and the external review provided by the US Food and Drug Administration all provide maximally efficient and robust utilization of the data. The CANVAS Program should significantly advance our understanding of the effects of canagliflozin, and the broader SGLT2 inhibitor class, on a range of important efficacy and safety outcomes.
Keywords: SGLT2 inhibitor; cardiovascular disease; type 2 diabetes.
© 2017 The Authors. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Figures

References
-
- Janssen Research & Development, LLC . Endocrinologic and Metabolic Drugs Advisory Committee. Canagliflozin as an adjunctive treatment to diet and exercise alone or co‐administered with other antihyperglycemic agents to improve glycemic control in adults with type 2 diabetes mellitus. JNJ‐28431754 (Canagliflozin). NDA 204042. January 10, 2013. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMateria.... Accessed December 21, 2016.
-
- Neal B, Perkovic V, de Zeeuw D, et al. Rationale, design, and baseline characteristics of the CANagliflozin cardioVascular Assessment Study (CANVAS)—a randomized placebo‐controlled trial. Am Heart J. 2013;166(2):217‐223. - PubMed
-
- Janssen Research & Development , LLC. Evaluation of the effects of canagliflozin on renal and cardiovascular outcomes in participants with diabetic nephropathy (CREDENCE). ClinicalTrials.gov Identifier: NCT02065791. February 17, 2014. https://clinicaltrials.gov/ct2/show/NCT02065791. Accessed December 19, 2016.
-
- Wu JH, Foote C, Blomster J, et al. Effects of sodium‐glucose cotransporter‐2 inhibitors on cardiovascular events, death, and major safety outcomes in adults with type 2 diabetes: a systematic review and meta‐analysis. Lancet Diabetes Endocrinol. 2016;4(5):411‐419. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

