Nandrolone decanoate interferes with testosterone biosynthesis altering blood-testis barrier components
- PMID: 28244681
- PMCID: PMC5542904
- DOI: 10.1111/jcmm.13092
Nandrolone decanoate interferes with testosterone biosynthesis altering blood-testis barrier components
Abstract
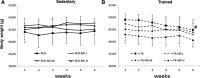
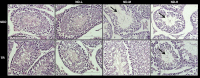
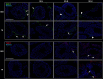
The aim of this study was to investigate whether nandrolone decanoate (ND) use affects testosterone production and testicular morphology in a model of trained and sedentary mice. A group of mice underwent endurance training while another set led a sedentary lifestyle and were freely mobile within cages. All experimental groups were treated with either ND or peanut oil at different doses for 6 weeks. Testosterone serum levels were measured via liquid chromatography-mass spectrometry. Western blot analysis and quantitative real-time PCR were utilized to determine gene and protein expression levels of the primary enzymes implicated in testosterone biosynthesis and gene expression levels of the blood-testis barrier (BTB) components. Immunohistochemistry and immunofluorescence were conducted for testicular morphological evaluation. The study demonstrated that moderate to high doses of ND induced a diminished serum testosterone level and altered the expression level of the key steroidogenic enzymes involved in testosterone biosynthesis. At the morphological level, ND induced degradation of the BTB by targeting the tight junction protein-1 (TJP1). ND stimulation deregulated metalloproteinase-9, metalloproteinase-2 (MMP-2) and the tissue inhibitor of MMP-2. Moreover, ND administration resulted in a mislocalization of mucin-1. In conclusion, ND abuse induces a decline in testosterone production that is unable to regulate the internalization and redistribution of TJP1 and may induce the deregulation of other BTB constituents via the inhibition of MMP-2. ND may well be considered as both a potential inducer of male infertility and a potential risk factor to a low endogenous bioavailable testosterone.
Keywords: MMP-2; MMP-9; MUC1; TJP1; blood-testis barrier; nandrolone decanoate; testosterone.
© 2017 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Figures




References
-
- Nilsson S, Allebeck P, Marklund B, et al Evaluation of a health promotion programme to prevent the misuse of androgenic anabolic steroids among Swedish adolescents. Health Promot Int. 2004; 19: 61–7. - PubMed
-
- Kostic TS, Stojkov NJ, Bjelic MM, et al Pharmacological doses of testosterone upregulated androgen receptor and 3‐Beta‐hydroxysteroid dehydrogenase/delta‐5‐delta‐4 isomerase and impaired leydig cells steroidogenesis in adult rats. Toxicol Sci. 2011; 121: 397–407. - PubMed
-
- Janjic MM, Stojkov NJ, Andric SA, et al Anabolic‐androgenic steroids induce apoptosis and NOS2 (nitric‐oxide synthase 2) in adult rat Leydig cells following in vivo exposure. Reprod Toxicol. 2012; 34: 686–93. - PubMed
-
- Wu X, Wan S, Lee MM. Key factors in the regulation of fetal and postnatal Leydig cell development. J Cell Physiol. 2007; 213: 429–33. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

