Therapeutic potential of adipose-derived stem cells and macrophages for ischemic skeletal muscle repair
- PMID: 28244825
- PMCID: PMC5348723
- DOI: 10.2217/rme-2016-0094
Therapeutic potential of adipose-derived stem cells and macrophages for ischemic skeletal muscle repair
Abstract
Aim: Progressive ischemia due to peripheral artery disease causes muscle damage and reduced strength of the lower extremities. Autologous cell therapy is an attractive treatment to restore perfusion and improve muscle function. Adipose-derived stem cells (ASCs) have therapeutic potential in tissue repair, including polarizing effects on macrophages (MPs).
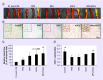
Materials & methods: Co-culture systems of ASCs and MPs were analyzed for gene and protein expression modifications in ASC-conditioned MPs. Co-transplantation of MPs/ASCs in vivo led to improved skeletal muscle regeneration in a mouse model of peripheral artery disease.
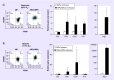
Results: ASCs/MPs therapy restored muscle function, increased perfusion and reduced inflammatory infiltrate.
Conclusion: Combined MPs/ASCs cell therapy is a promising approach to restore muscle function and stimulate local angiogenesis in the ischemic limb.
Keywords: adipose-derived stem cells; cell-mediated therapy; ischemic injury; macrophages; peripheral artery disease; skeletal muscle regeneration.
Conflict of interest statement
This research was supported by grants from NIH #R01EB015007 and American Heart Association #15GRNT22960026). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Figures





References
-
- Criqui MH, Aboyans V. Epidemiology of peripheral artery disease. Circ. Res. 2015;116(9):1509–1526. - PubMed
-
- Criqui MH, Denenberg JO, Langer RD, Fronek A. The epidemiology of peripheral arterial disease: importance of identifying the population at risk. Vasc. Med. 1997;2(3):221–226. - PubMed
-
- Fowkes FG, Rudan D, Rudan I, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382(9901):1329–1340. - PubMed
-
- Serrano Hernando FJ, Martin Conejero A. Peripheral artery disease: pathophysiology, diagnosis and treatment. Rev. Esp. Cardiol. 2007;60(9):969–982. - PubMed
-
- Bird CE, Criqui MH, Fronek A, Denenberg JO, Klauber MR, Langer RD. Quantitative and qualitative progression of peripheral arterial disease by non-invasive testing. Vasc. Med. 1999;4(1):15–21. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
