Lipid droplet biology and evolution illuminated by the characterization of a novel perilipin in teleost fish
- PMID: 28244868
- PMCID: PMC5342826
- DOI: 10.7554/eLife.21771
Lipid droplet biology and evolution illuminated by the characterization of a novel perilipin in teleost fish
Abstract
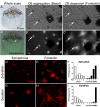
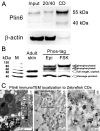
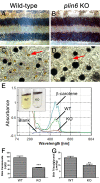
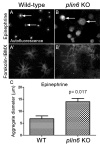
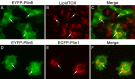
Perilipin (PLIN) proteins constitute an ancient family important in lipid droplet (LD) formation and triglyceride metabolism. We identified an additional PLIN clade (plin6) that is unique to teleosts and can be traced to the two whole genome duplications that occurred early in vertebrate evolution. Plin6 is highly expressed in skin xanthophores, which mediate red/yellow pigmentation and trafficking, but not in tissues associated with lipid metabolism. Biochemical and immunochemical analyses demonstrate that zebrafish Plin6 protein targets the surface of pigment-containing carotenoid droplets (CD). Protein kinase A (PKA) activation, which mediates CD dispersion in xanthophores, phosphorylates Plin6 on conserved residues. Knockout of plin6 in zebrafish severely impairs the ability of CD to concentrate carotenoids and prevents tight clustering of CD within carotenoid bodies. Ultrastructural and functional analyses indicate that LD and CD are homologous structures, and that Plin6 was functionalized early in vertebrate evolution for concentrating and trafficking pigment.
Keywords: carotenoid; cell biology; evolutionary biology; genomics; lipid; perilipin; spotted gar; teleost; xanthophore; zebrafish.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures









References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

