The chemical evolution of oligonucleotide therapies of clinical utility
- PMID: 28244990
- PMCID: PMC5517098
- DOI: 10.1038/nbt.3765
The chemical evolution of oligonucleotide therapies of clinical utility
Abstract
After nearly 40 years of development, oligonucleotide therapeutics are nearing meaningful clinical productivity. One of the key advantages of oligonucleotide drugs is that their delivery and potency are derived primarily from the chemical structure of the oligonucleotide whereas their target is defined by the base sequence. Thus, as oligonucleotides with a particular chemical design show appropriate distribution and safety profiles for clinical gene silencing in a particular tissue, this will open the door to the rapid development of additional drugs targeting other disease-associated genes in the same tissue. To achieve clinical productivity, the chemical architecture of the oligonucleotide needs to be optimized with a combination of sugar, backbone, nucleobase, and 3'- and 5'-terminal modifications. A portfolio of chemistries can be used to confer drug-like properties onto the oligonucleotide as a whole, with minor chemical changes often translating into major improvements in clinical efficacy. One outstanding challenge in oligonucleotide chemical development is the optimization of chemical architectures to ensure long-term safety. There are multiple designs that enable effective targeting of the liver, but a second challenge is to develop architectures that enable robust clinical efficacy in additional tissues.
Figures



 Gray, 2′-F; ● Black, 2′-OMe or 2′-MOE;
Gray, 2′-F; ● Black, 2′-OMe or 2′-MOE;
 Blue, LNA or cEt,
Blue, LNA or cEt,
 Green, specificity enhancing modification; red, phosphorothioate backbone modification (direction of the bond indicates positional stereopurity Rp or Sp). PUFA, polyunsaturated fatty acids; gen 2, second generation.
Green, specificity enhancing modification; red, phosphorothioate backbone modification (direction of the bond indicates positional stereopurity Rp or Sp). PUFA, polyunsaturated fatty acids; gen 2, second generation.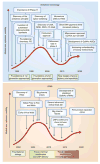
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

