The Regulations of Deubiquitinase USP15 and Its Pathophysiological Mechanisms in Diseases
- PMID: 28245560
- PMCID: PMC5372499
- DOI: 10.3390/ijms18030483
The Regulations of Deubiquitinase USP15 and Its Pathophysiological Mechanisms in Diseases
Erratum in
-
Correction: Chon-Kit Chou, et al. The Regulations of Deubiquitinase USP15 and Its Pathophysiological Mechanisms in Diseases. Int. J. Mol. Sci. 2017, 18, 483.Int J Mol Sci. 2017 Apr 25;18(5):902. doi: 10.3390/ijms18050902. Int J Mol Sci. 2017. PMID: 28441349 Free PMC article.
Abstract
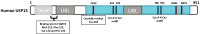
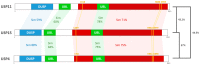
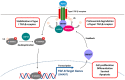
Deubiquitinases (DUBs) play a critical role in ubiquitin-directed signaling by catalytically removing the ubiquitin from substrate proteins. Ubiquitin-specific protease 15 (USP15), a member of the largest subfamily of cysteine protease DUBs, contains two conservative cysteine (Cys) and histidine (His) boxes. USP15 harbors two zinc-binding motifs that are essential for recognition of poly-ubiquitin chains. USP15 is grouped into the same category with USP4 and USP11 due to high degree of homology in an N-terminal region consisting of domains present in ubiquitin-specific proteases (DUSP) domain and ubiquitin-like (UBL) domain. USP15 cooperates with COP9 signalosome complex (CSN) to maintain the stability of cullin-ring ligase (CRL) adaptor proteins by removing the conjugated ubiquitin chains from RBX1 subunit of CRL. USP15 is also implicated in the stabilization of the human papillomavirus type 16 E6 oncoprotein, adenomatous polyposis coli, and IκBα. Recently, reports have suggested that USP15 acts as a key regulator of TGF-β receptor-signaling pathways by deubiquitinating the TGF-β receptor itself and its downstream transducers receptor-regulated SMADs (R-SMADs), including SMAD1, SMAD2, and SMAD3, thus activating the TGF-β target genes. Although the importance of USP15 in pathologic processes remains ambiguous so far, in this review, we endeavor to summarize the literature regarding the relationship of the deubiquitinating action of USP15 with the proteins involved in the regulation of Parkinson's disease, virus infection, and cancer-related signaling networks.
Keywords: IκBα; Parkinson’s disease; TGF-β; cancer-related signaling networks; deubiquitinase; ubiquitin-specific protease 15.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

