Pancreatic Cancer-Induced Neutrophil Extracellular Traps: A Potential Contributor to Cancer-Associated Thrombosis
- PMID: 28245569
- PMCID: PMC5372503
- DOI: 10.3390/ijms18030487
Pancreatic Cancer-Induced Neutrophil Extracellular Traps: A Potential Contributor to Cancer-Associated Thrombosis
Abstract
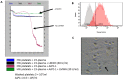
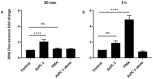
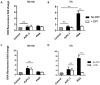
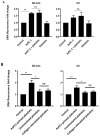
Pancreatic cancer (PaCa) is a highly metastatic cancer, and patients are at high risk of developing venous thromboembolism (VTE). Neutrophil extracellular traps (NETs) have been associated with cancer metastasis and cancer-associated thrombosis, but the ability of cancer to stimulate NET release is not known. The release of NETs has been shown to be a slow process and requires reactive oxygen species (ROS) production. Studies suggest that activated platelets are important mediators in the release. Here, we show that PaCa cells can stimulate the rapid release of NETs, independently of ROS production. We further assessed the role of platelets in PaCa-induced NETs and observed a trend of increased the NET release by PaCa-primed platelets. Additionally, NETs promoted thrombus formation under venous shear stress ex vivo. Taken together, our results suggest that PaCa-induced NETs can contribute to the high risk of venous thromboembolism development in PaCa patients, and reveal NETs as a potential therapeutic target.
Keywords: neutrophil extracellular traps; pancreatic cancer; platelets; venous thromboembolism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


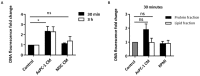



References
-
- Yoo D.-G., Winn M., Pang L., Moskowitz S.M., Malech H.L., Leto T.L., Rada B. Release of cystic fibrosis airway inflammatory markers from pseudomonas aeruginosa-stimulated human neutrophils involves nadph oxidase-dependent extracellular DNA trap formation. J. Immunol. 2014;192:4728–4738. doi: 10.4049/jimmunol.1301589. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

