Influence of DPH1 and DPH5 Protein Variants on the Synthesis of Diphthamide, the Target of ADPRibosylating Toxins
- PMID: 28245596
- PMCID: PMC5371833
- DOI: 10.3390/toxins9030078
Influence of DPH1 and DPH5 Protein Variants on the Synthesis of Diphthamide, the Target of ADPRibosylating Toxins
Abstract
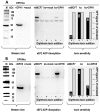
The diphthamide on eukaryotic translation elongation factor 2 (eEF2) is the target of ADPribosylating toxins and -derivatives that serve as payloads in targeted tumor therapy. Diphthamide is generated by seven DPH proteins; cells deficient in these (DPHko) lack diphthamide and are toxin-resistant. We have established assays to address the functionality of DPH1 (OVCA1) and DPH5 variants listed in dbSNP and cosmic databases: plasmids encoding wildtype and mutant DPHs were transfected into DPHko cells. Supplementation of DPH1 and DPH5 restores diphthamide synthesis and toxin sensitivity in DPH1ko and DPH5ko cells, respectively. Consequently, the determination of the diphthamide status of cells expressing DPH variants differentiates active and compromised proteins. The DPH1 frameshift variant L96fs* (with Nterminal 96 amino acids, truncated thereafter) and two splice isoforms lacking 80 or 140 amino acids at their N-termini failed to restore DPH1ko deficiency. The DPH1 frameshift variant R312fs* retained some residual activity even though it lacks a large C-terminal portion. DPH1 missense variants R27W and S56F retained activity while S221P had reduced activity, indicated by a decreased capability to restore diphthamide synthesis. The DPH5 nonsense or frameshift variants E60*, W136fs* and R207* (containing intact N-termini with truncations after 60, 136 or 207 amino acids, respectively) were inactive: none compensated the deficiency of DPH5ko cells. In contrast, missense variants D57G, G87R, S123C and Q170H as well as the frequently occurring DPH5 isoform delA212 retained activity. Sensitivity to ADP-ribosylating toxins and tumor-targeted immunotoxins depends on diphthamide which, in turn, requires DPH functionality. Because of that, DPH variants (in particular those that are functionally compromised) may serve as a biomarker and correlate with the efficacy of immunotoxin-based therapies.
Keywords: pseudomonas exotoxin; biomarker; OVCA1; diphtheria toxin; immunotoxin; targeted therapy.
Conflict of interest statement
The authors are employed by and part of Roche Pharma Research & Early Development. Roche has an interest in the development of targeted therapies.
Figures



References
-
- Stahl S., Mueller F., Pastan I., Brinkmann U. Factors that Determine Sensitivity and Resistances of Tumor Cells towards Antibody-Targeted Protein Toxins. In: Verma R.S., Bonavida B., editors. Resistance to Immunotoxins in Cancer Therapy. Springer International Publishing; Cham, Switzerland: 2015.
-
- Liu S., Milne G.T., Kuremsky J.G., Fink G.R., Leppla S.H. Identification of the proteins required for biosynthesis of diphthamide, the target of bacterial ADP-ribosylating toxins on translation elongation factor 2. Mol. Cell. Biol. 2004;24:9487–9497. doi: 10.1128/MCB.24.21.9487-9497.2004. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

