Macrocyclic Antimicrobial Peptides Engineered from ω-Conotoxin
- PMID: 28245769
- PMCID: PMC5470054
- DOI: 10.2174/1381612822666161027120518
Macrocyclic Antimicrobial Peptides Engineered from ω-Conotoxin
Abstract
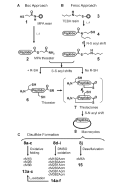
The potent calcium channel blocker ω-conotoxin MVIIA is a linear cystine-knot peptide with multiple basic amino acids at both termini. This work shows that macrocyclization of MVIIA linking two positive-charge terminal clusters as a contiguous segment converts a conotoxin into an antimicrobial peptide. In addition, conversion of disulfide bonds to amino butyric acids improved the antimicrobial activity of the cyclic analogs. Ten macrocyclic analogs, with or without disulfide bonds, were prepared by both Boc and Fmoc chemistry using native chemical ligation. All cyclic analogs were active against selected Gram-positive and Gram-negative bacteria with minimal inhibitory concentrations in a low μM range. In contrast, MVIIA and its linear analog were inactive at concentrations up to 0.5 mM. The cyclic analogs also showed 2 to 3-fold improved chemotactic activity against human monocytes THP-1 compared with MVIIA. Reduction of molecular stability against thermal and acid treatment due to the reduced number of disulfide crosslinks can be partly restored by backbone cyclization. Together, these results show that macrocyclization and side chain modification of a linear conopeptide lead to a gain-of-function, which brings a new perspective in designing and engineering of peptidyl therapeutics.
Keywords: Macrocyclization; antimicrobial peptide; chemotaxis.; cyclic conotoxin.
Copyright© Bentham Science Publishers; For any queries, please email at epub@benthamscience.org.
Figures
Similar articles
-
An unusual structural motif of antimicrobial peptides containing end-to-end macrocycle and cystine-knot disulfides.Proc Natl Acad Sci U S A. 1999 Aug 3;96(16):8913-8. doi: 10.1073/pnas.96.16.8913. Proc Natl Acad Sci U S A. 1999. PMID: 10430870 Free PMC article.
-
Synthesis and biological activity of lipophilic analogs of the cationic antimicrobial active peptide anoplin.J Pept Sci. 2016 Nov;22(11-12):731-736. doi: 10.1002/psc.2939. Epub 2016 Nov 15. J Pept Sci. 2016. PMID: 27862650
-
Design and membrane-disruption mechanism of charge-enriched AMPs exhibiting cell selectivity, high-salt resistance, and anti-biofilm properties.Amino Acids. 2016 Feb;48(2):505-22. doi: 10.1007/s00726-015-2104-0. Epub 2015 Oct 8. Amino Acids. 2016. PMID: 26450121
-
Antimicrobial AApeptides.Curr Top Med Chem. 2017;17(11):1266-1279. doi: 10.2174/1568026616666161018145945. Curr Top Med Chem. 2017. PMID: 27758686 Free PMC article. Review.
-
Structure-activity relationships of omega-conotoxins at N-type voltage-sensitive calcium channels.J Mol Recognit. 2000 Mar-Apr;13(2):55-70. doi: 10.1002/(SICI)1099-1352(200003/04)13:2<55::AID-JMR488>3.0.CO;2-O. J Mol Recognit. 2000. PMID: 10822250 Review.
Cited by
-
A Chemoenzymatic Approach To Produce a Cyclic Analogue of the Analgesic Drug MVIIA (Ziconotide).Angew Chem Int Ed Engl. 2023 Jul 17;62(29):e202302812. doi: 10.1002/anie.202302812. Epub 2023 May 31. Angew Chem Int Ed Engl. 2023. PMID: 37148162 Free PMC article.
-
Combined Proteotranscriptomic-Based Strategy to Discover Novel Antimicrobial Peptides from Cone Snails.Biomedicines. 2021 Mar 29;9(4):344. doi: 10.3390/biomedicines9040344. Biomedicines. 2021. PMID: 33805497 Free PMC article. Review.
-
Redesigning Arenicin-1, an Antimicrobial Peptide from the Marine Polychaeta Arenicola marina, by Strand Rearrangement or Branching, Substitution of Specific Residues, and Backbone Linearization or Cyclization.Mar Drugs. 2019 Jun 23;17(6):376. doi: 10.3390/md17060376. Mar Drugs. 2019. PMID: 31234579 Free PMC article.
-
Design of Potent and Salt-Insensitive Antimicrobial Branched Peptides.Polymers (Basel). 2023 Aug 29;15(17):3594. doi: 10.3390/polym15173594. Polymers (Basel). 2023. PMID: 37688220 Free PMC article.
-
Molluscan Compounds Provide Drug Leads for the Treatment and Prevention of Respiratory Disease.Mar Drugs. 2020 Nov 19;18(11):570. doi: 10.3390/md18110570. Mar Drugs. 2020. PMID: 33228163 Free PMC article. Review.
References
-
- Hruby V. Conformational restrictions of biologically active peptides via amino acid side chain groups. Life Sci. 1982;31:189–199. - PubMed
-
- Wong C., Rowlands D., Wong C-H., et al. Orally Active Peptidic Bradykinin B1 Receptor Antagonists Engineered from a Cyclotide Scaffold for Inflammatory Pain Treatment. Angew. Chem. Int. Ed. 2012;51:5620–5624. - PubMed
-
- Verdine G., Hilinski G. Chapter one - Stapled Peptides for Intracellular Drug Targets.Methods enzymol. Academic Press, 2012. - PubMed
-
- Baeriswyl V., Heinis C. Polycyclic peptide therapeutics. Chem. Med. Chem. 2013;8:377–384. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
