Characterization of two novel intronic OPA1 mutations resulting in aberrant pre-mRNA splicing
- PMID: 28245802
- PMCID: PMC5331656
- DOI: 10.1186/s12881-017-0383-x
Characterization of two novel intronic OPA1 mutations resulting in aberrant pre-mRNA splicing
Abstract
Background: We report two novel splice region mutations in OPA1 in two unrelated families presenting with autosomal-dominant optic atrophy type 1 (ADOA1) (ADOA or Kjer type optic atrophy). Mutations in OPA1 encoding a mitochondrial inner membrane protein are a major cause of ADOA.
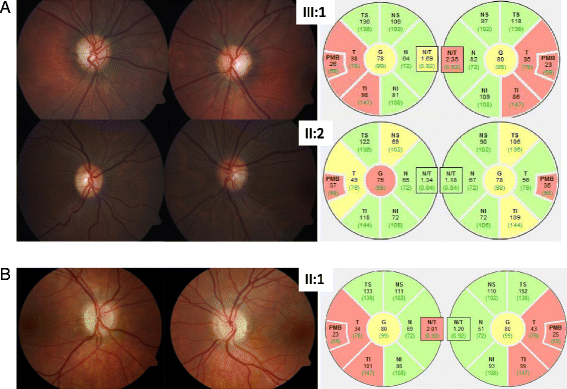
Methods: We analyzed two unrelated families including four affected individuals clinically suspicious of ADOA. Standard ocular examinations were performed in affected individuals of both families. All coding exons, as well as exon-intron boundaries of the OPA1 gene were sequenced. In addition, multiplex ligation-dependent probe amplification (MLPA) was performed to uncover copy number variations in OPA1. mRNA processing was monitored using RT-PCR and subsequent cDNA analysis.
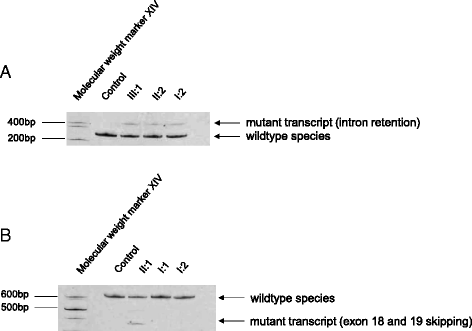
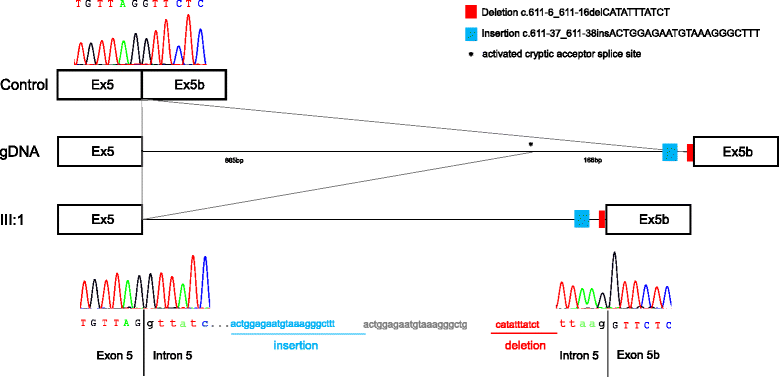
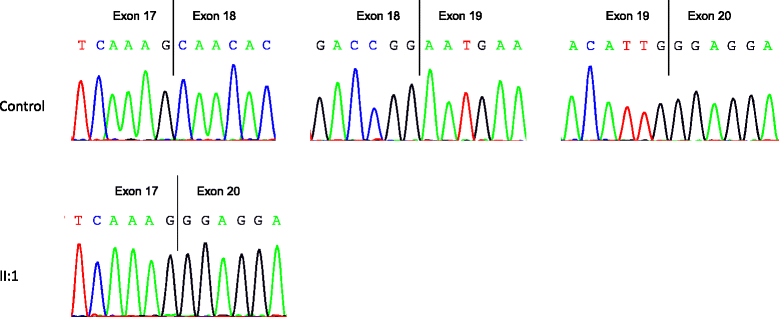
Results: We report two novel splice region mutations in OPA1 in two unrelated individuals and their affected relatives, which were previously not described in the literature. In one family the heterozygous insertion and deletion c.[611-37_611-38insACTGGAGAATGTAAAGGGCTTT;611-6_611-16delCATATTTATCT] was found in all investigated family members leading to the activation of an intronic cryptic splice site. In the second family sequencing of OPA1 disclosed a de novo heterozygous deletion c.2012+4_2012+7delAGTA resulting in exon 18 and 19 skipping, which was not detected in healthy family members.
Conclusion: We identified two novel intronic mutations in OPA1 affecting the correct OPA1 pre-mRNA splicing, which was confirmed by OPA1 cDNA analysis. This study shows the importance of transcript analysis to determine the consequences of unclear intronic mutations in OPA1 in proximity to the intron-exon boundaries.
Keywords: Autosomal dominant optic atrophy; Kjer type optic atrophy; OPA1; Optic neuropathies; Splice site mutation.
Figures

Similar articles
-
A comprehensive survey of mutations in the OPA1 gene in patients with autosomal dominant optic atrophy.Invest Ophthalmol Vis Sci. 2002 Jun;43(6):1715-24. Invest Ophthalmol Vis Sci. 2002. PMID: 12036970
-
Dominant optic atrophy caused by a novel OPA1 splice site mutation (IVS20+1G-->A) associated with intron retention.Ophthalmic Res. 2005 Jul-Aug;37(4):214-24. doi: 10.1159/000086862. Epub 2005 Jul 5. Ophthalmic Res. 2005. PMID: 16006781
-
Activation of cryptic splice sites is a frequent splicing defect mechanism caused by mutations in exon and intron sequences of the OPA1 gene.Hum Genet. 2006 Feb;118(6):767-71. doi: 10.1007/s00439-005-0096-7. Epub 2005 Dec 2. Hum Genet. 2006. PMID: 16323009
-
Recessive optic atrophy, sensorimotor neuropathy and cataract associated with novel compound heterozygous mutations in OPA1.Mol Med Rep. 2016 Jul;14(1):33-40. doi: 10.3892/mmr.2016.5209. Epub 2016 May 4. Mol Med Rep. 2016. PMID: 27150940 Free PMC article. Review.
-
Meta-analysis of genotype-phenotype analysis of OPA1 mutations in autosomal dominant optic atrophy.Mitochondrion. 2019 May;46:262-269. doi: 10.1016/j.mito.2018.07.006. Epub 2018 Aug 27. Mitochondrion. 2019. PMID: 30165240
Cited by
-
First submicroscopic inversion of the OPA1 gene identified in dominant optic atrophy - a case report.BMC Med Genet. 2020 Nov 26;21(1):236. doi: 10.1186/s12881-020-01166-z. BMC Med Genet. 2020. PMID: 33243194 Free PMC article.
References
-
- Kjer P. Infantile optic atrophy with dominant mode of inheritance: a clinical and genetic study of 19 Danish families. Acta Ophthalmol Suppl. 1959;164(Supp 54):1–147. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

