Epstein-Barr virus lytic infection promotes activation of Toll-like receptor 8 innate immune response in systemic sclerosis monocytes
- PMID: 28245863
- PMCID: PMC5331713
- DOI: 10.1186/s13075-017-1237-9
Epstein-Barr virus lytic infection promotes activation of Toll-like receptor 8 innate immune response in systemic sclerosis monocytes
Abstract
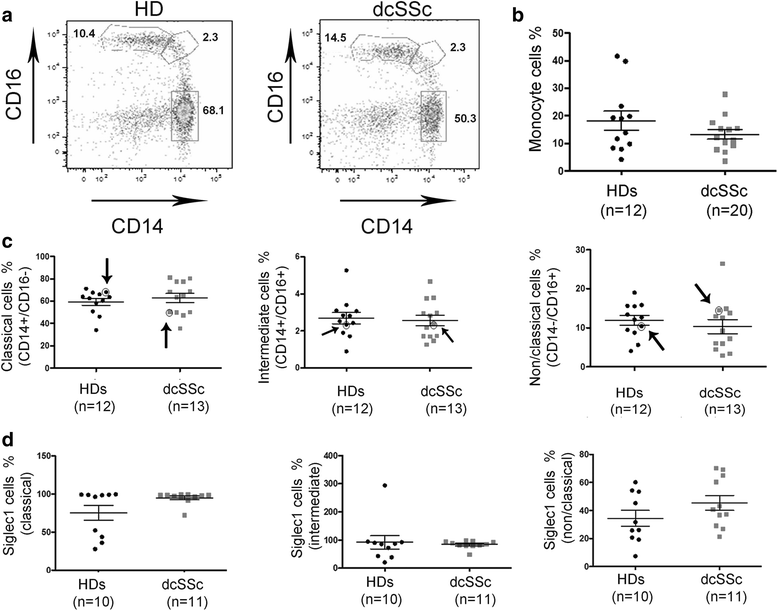
Background: Monocytes/macrophages are activated in several autoimmune diseases, including systemic sclerosis (scleroderma; SSc), with increased expression of interferon (IFN)-regulatory genes and inflammatory cytokines, suggesting dysregulation of the innate immune response in autoimmunity. In this study, we investigated whether the lytic form of Epstein-Barr virus (EBV) infection (infectious EBV) is present in scleroderma monocytes and contributes to their activation in SSc.
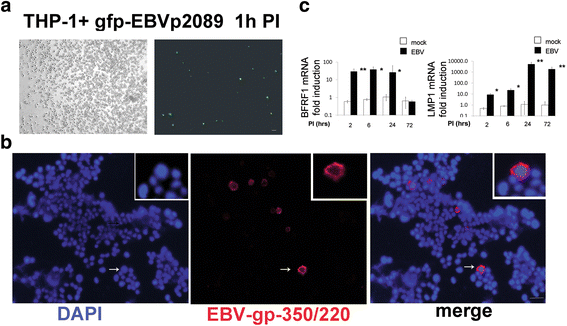
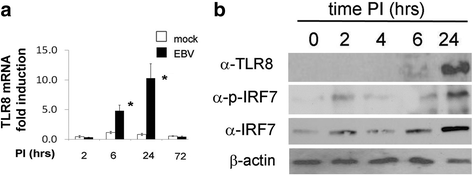
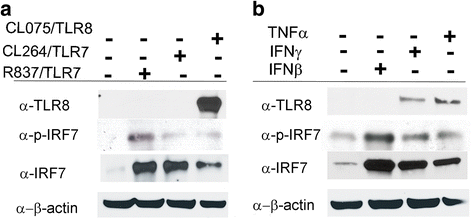
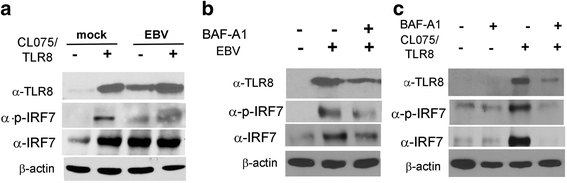
Methods: Monocytes were isolated from peripheral blood mononuclear cells (PBMCs) depleted of the CD19+ cell fraction, using CD14/CD16 negative-depletion. Circulating monocytes from SSc and healthy donors (HDs) were infected with EBV. Gene expression of innate immune mediators were evaluated in EBV-infected monocytes from SSc and HDs. Involvement of Toll-like receptor (TLR)8 in viral-mediated TLR8 response was investigated by comparing the TLR8 expression induced by infectious EBV to the expression stimulated by CL075/TLR8/agonist-ligand in the presence of TLR8 inhibitor in THP-1 cells.
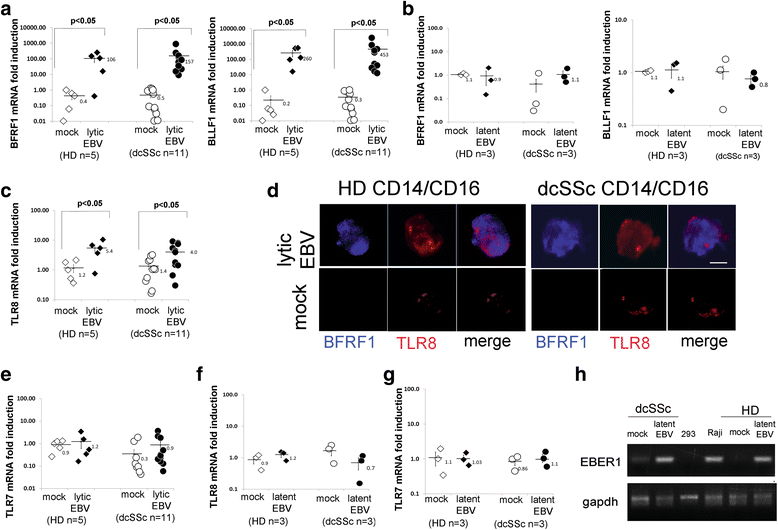
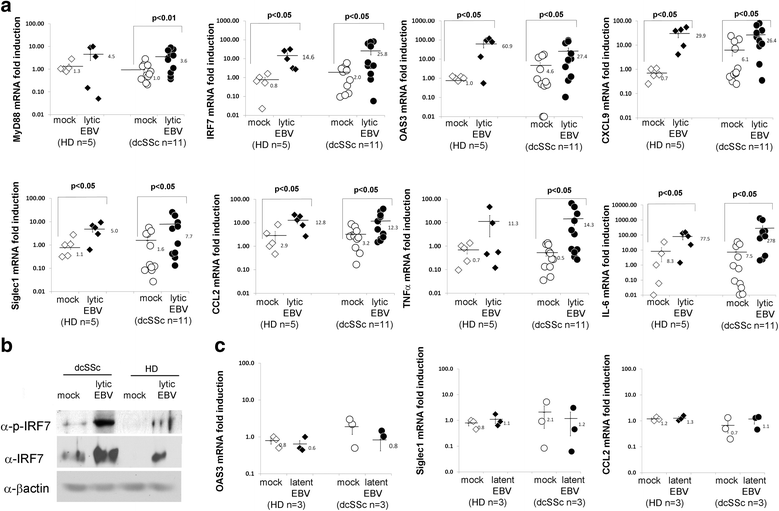
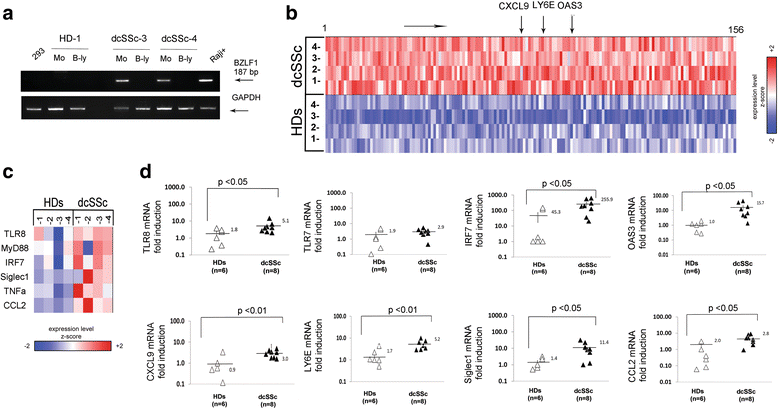
Results: Infectious EBV strongly induced TLR8 expression in infected SSc and HD monocytes in vitro. Markers of activated monocytes, such as IFN-regulated genes and chemokines, were upregulated in SSc- and HD-EBV-infected monocytes. Inhibiting TLR8 expression reduced virally induced TLR8 in THP-1 infected cells, demonstrating that innate immune activation by infectious EBV is partially dependent on TLR8. Viral mRNA and proteins were detected in freshly isolated SSc monocytes. Microarray analysis substantiated the evidence of an increased IFN signature and altered level of TLR8 expression in SSc monocytes carrying infectious EBV compared to HD monocytes.
Conclusion: This study provides the first evidence of infectious EBV in monocytes from patients with SSc and links EBV to the activation of TLR8 and IFN innate immune response in freshly isolated SSc monocytes. This study provides the first evidence of EBV replication activating the TLR8 molecular pathway in primary monocytes. Immunogenicity of infectious EBV suggests a novel mechanism mediating monocyte inflammation in SSc, by which EBV triggers the innate immune response in infected cells.
Keywords: EBV reactivation; IFN inducible genes; Innate immune response; Monocytes; Systemic sclerosis; Toll-like receptor 8.
Figures

Similar articles
-
Innate Immune Modulation Induced by EBV Lytic Infection Promotes Endothelial Cell Inflammation and Vascular Injury in Scleroderma.Front Immunol. 2021 Apr 19;12:651013. doi: 10.3389/fimmu.2021.651013. eCollection 2021. Front Immunol. 2021. PMID: 33953718 Free PMC article.
-
Immune activation suppresses initiation of lytic Epstein-Barr virus infection.Cell Microbiol. 2007 Aug;9(8):2055-69. doi: 10.1111/j.1462-5822.2007.00937.x. Epub 2007 Apr 5. Cell Microbiol. 2007. PMID: 17419714
-
A macrophage marker, Siglec-1, is increased on circulating monocytes in patients with systemic sclerosis and induced by type I interferons and toll-like receptor agonists.Arthritis Rheum. 2007 Mar;56(3):1010-20. doi: 10.1002/art.22382. Arthritis Rheum. 2007. PMID: 17328080
-
Regulation and dysregulation of Epstein-Barr virus latency: implications for the development of autoimmune diseases.Autoimmunity. 2008 May;41(4):298-328. doi: 10.1080/08916930802024772. Autoimmunity. 2008. PMID: 18432410 Review.
-
[Regulation and evasion of host immune responses by Epstein-Barr virus].Wei Sheng Wu Xue Bao. 2016 Jan 4;56(1):19-25. Wei Sheng Wu Xue Bao. 2016. PMID: 27305776 Review. Chinese.
Cited by
-
Anti-viral and pro-inflammatory functions of Toll-like receptors during gamma-herpesvirus infections.Virol J. 2021 Nov 8;18(1):218. doi: 10.1186/s12985-021-01678-x. Virol J. 2021. PMID: 34749760 Free PMC article. Review.
-
Autoimmune/inflammatory syndrome induced by adjuvants-ASIA-related to biomaterials: analysis of 45 cases and comprehensive review of the literature.Immunol Res. 2018 Feb;66(1):120-140. doi: 10.1007/s12026-017-8980-5. Immunol Res. 2018. PMID: 29199390
-
CD169 and CD64 could help differentiate bacterial from CoVID-19 or other viral infections in the Emergency Department.Cytometry A. 2021 May;99(5):435-445. doi: 10.1002/cyto.a.24314. Epub 2021 Feb 8. Cytometry A. 2021. PMID: 33491921 Free PMC article.
-
Significant up-regulation of Toll-like receptor (TLR) signaling pathway in Epstein-Barr virus-associated gastric cancer.Int J Mol Epidemiol Genet. 2025 Feb 25;16(1):1-8. doi: 10.62347/RIOX7768. eCollection 2025. Int J Mol Epidemiol Genet. 2025. PMID: 40151299 Free PMC article.
-
Molecular mimicry, genetic homology, and gene sharing proteomic "molecular fingerprints" using an EBV (Epstein-Barr virus)-derived microarray as a potential diagnostic method in autoimmune disease.Immunol Res. 2018 Dec;66(6):686-695. doi: 10.1007/s12026-018-9045-0. Immunol Res. 2018. PMID: 30552620 Review.
References
-
- Farina GA, York MR, Di Marzio M, Collins CA, Meller S, Homey B, Rifkin IR, Marshak-Rothstein A, Radstake TR, Lafyatis R. Poly(I:C) drives type I IFN- and TGFbeta-mediated inflammation and dermal fibrosis simulating altered gene expression in systemic sclerosis. J Invest Dermatol. 2010;130(11):2583–93. doi: 10.1038/jid.2010.200. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

