TGF-β Family Signaling in Tumor Suppression and Cancer Progression
- PMID: 28246180
- PMCID: PMC5710110
- DOI: 10.1101/cshperspect.a022277
TGF-β Family Signaling in Tumor Suppression and Cancer Progression
Abstract
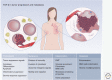
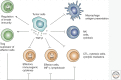
Transforming growth factor-β (TGF-β) induces a pleiotropic pathway that is modulated by the cellular context and its integration with other signaling pathways. In cancer, the pleiotropic reaction to TGF-β leads to a diverse and varied set of gene responses that range from cytostatic and apoptotic tumor-suppressive ones in early stage tumors, to proliferative, invasive, angiogenic, and oncogenic ones in advanced cancer. Here, we review the knowledge accumulated about the molecular mechanisms involved in the dual response to TGF-β in cancer, and how tumor cells evolve to evade the tumor-suppressive responses of this signaling pathway and then hijack the signal, converting it into an oncogenic factor. Only through the detailed study of this complexity can the suitability of the TGF-β pathway as a therapeutic target against cancer be evaluated.
Copyright © 2017 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures



References
-
- Adorno M, Cordenonsi M, Montagner M, Dupont S, Wong C, Hann B, Solari A, Bobisse S, Rondina MB, Guzzardo V, et al. 2009. A mutant-p53/Smad complex opposes p63 to empower TGFβ-induced metastasis. Cell 137: 87–98. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
