TGF-β Family Signaling in Epithelial Differentiation and Epithelial-Mesenchymal Transition
- PMID: 28246184
- PMCID: PMC5749157
- DOI: 10.1101/cshperspect.a022194
TGF-β Family Signaling in Epithelial Differentiation and Epithelial-Mesenchymal Transition
Abstract
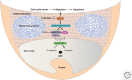
Epithelia exist in the animal body since the onset of embryonic development; they generate tissue barriers and specify organs and glands. Through epithelial-mesenchymal transitions (EMTs), epithelia generate mesenchymal cells that form new tissues and promote healing or disease manifestation when epithelial homeostasis is challenged physiologically or pathologically. Transforming growth factor-βs (TGF-βs), activins, bone morphogenetic proteins (BMPs), and growth and differentiation factors (GDFs) have been implicated in the regulation of epithelial differentiation. These TGF-β family ligands are expressed and secreted at sites where the epithelium interacts with the mesenchyme and provide paracrine queues from the mesenchyme to the neighboring epithelium, helping the specification of differentiated epithelial cell types within an organ. TGF-β ligands signal via Smads and cooperating kinase pathways and control the expression or activities of key transcription factors that promote either epithelial differentiation or mesenchymal transitions. In this review, we discuss evidence that illustrates how TGF-β family ligands contribute to epithelial differentiation and induce mesenchymal transitions, by focusing on the embryonic ectoderm and tissues that form the external mammalian body lining.
Copyright © 2018 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures


References
-
- Amendt C, Schirmacher P, Weber H, Blessing M. 1998. Expression of a dominant negative type II TGF-β receptor in mouse skin results in an increase in carcinoma incidence and an acceleration of carcinoma development. Oncogene 17: 25–34. - PubMed
-
- Andersson O, Bertolino P, Ibanez CF. 2007. Distinct and cooperative roles of mammalian Vg1 homologs GDF1 and GDF3 during early embryonic development. Dev Biol 311: 500–511. - PubMed
-
- Andl T, Reddy ST, Gaddapara T, Millar SE. 2002. WNT signals are required for the initiation of hair follicle development. Dev Cell 2: 643–653. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
