TGF-β Family Signaling in Connective Tissue and Skeletal Diseases
- PMID: 28246187
- PMCID: PMC5666637
- DOI: 10.1101/cshperspect.a022269
TGF-β Family Signaling in Connective Tissue and Skeletal Diseases
Abstract
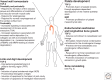
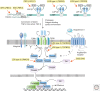
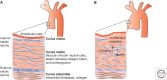
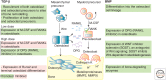
The transforming growth factor β (TGF-β) family of signaling molecules, which includes TGF-βs, activins, inhibins, and numerous bone morphogenetic proteins (BMPs) and growth and differentiation factors (GDFs), has important functions in all cells and tissues, including soft connective tissues and the skeleton. Specific TGF-β family members play different roles in these tissues, and their activities are often balanced with those of other TGF-β family members and by interactions with other signaling pathways. Perturbations in TGF-β family pathways are associated with numerous human diseases with prominent involvement of the skeletal and cardiovascular systems. This review focuses on the role of this family of signaling molecules in the pathologies of connective tissues that manifest in rare genetic syndromes (e.g., syndromic presentations of thoracic aortic aneurysm), as well as in more common disorders (e.g., osteoarthritis and osteoporosis). Many of these diseases are caused by or result in pathological alterations of the complex relationship between the TGF-β family of signaling mediators and the extracellular matrix in connective tissues.
Copyright © 2017 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures

References
-
- Ahn K, Mishina Y, Hanks MC, Behringer RR, Crenshaw EB III. 2001. BMPR-IA signaling is required for the formation of the apical ectodermal ridge and dorsal–ventral patterning of the limb. Development 128: 4449–4461. - PubMed
-
- Akiyama H. 2008. Control of chondrogenesis by the transcription factor Sox9. Mod Rheumatol 18: 213–219. - PubMed
-
- Akiyoshi S, Inoue H, Hanai J, Kusanagi K, Nemoto N, Miyazono K, Kawabata M. 1999. c-Ski acts as a transcriptional co-repressor in transforming growth factor-β signaling through interaction with Smads. J Biol Chem 274: 35269–35277. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
